Discovery of a novel CYP107E41 from Streptoalloteichus sp. KCCM40925 for advancing steroid hydroxylation catalysis
IF 3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Cytochrome P450 enzymes (CYPs) are versatile biocatalysts involved in the biosynthesis, activation, and detoxification of a wide range of endogenous and exogenous compounds. While the CYP107 family is primarily associated with macrolide antibiotic biosynthesis, some members, such as OleP (CYP107D1) and CYP107X1, have also been shown to catalyze steroid hydroxylation, albeit with limited substrate scope. In this study, we identified CYP107E41 from Streptoalloteichus sp. KCCM40925 as a novel member of the CYP107 family with selective hydroxylation activity toward both androstane and pregnane steroids. In-vitro screening using a panel of eleven steroids revealed predominant 6β- and 16α-hydroxylation, with androstane substrates exhibiting higher conversion efficiencies. Among the redox systems evaluated, diacetoxyiodobenzene provided the highest catalytic efficiency. Molecular docking identified two major binding orientations, C6–Fe and C16–Fe, with the C6–Fe configuration consistently scoring higher in CNN pose evaluations, suggesting it as the catalytically preferred binding mode. Molecular dynamics simulations further supported this, showing that the testosterone complex maintained a stable conformation with C6 positioned near the heme iron, consistent with selective mono-hydroxylation. In contrast, the nandrolone complex exhibited greater conformational flexibility, maintaining the proximity of both C6 and C7 to the heme, aligning with its broader hydroxylation profile. These findings establish CYP107E41 as a promising candidate for regioselective steroid hydroxylation and provide mechanistic insights to inform future protein engineering and redox system optimization.
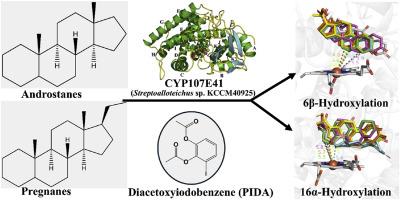
从Streptoalloteichus sp. KCCM40925中发现新的CYP107E41促进类固醇羟基化催化
细胞色素P450酶(CYPs)是一种多用途的生物催化剂,参与多种内源性和外源性化合物的生物合成、活化和解毒。虽然CYP107家族主要与大环内酯类抗生素的生物合成有关,但一些成员,如OleP (CYP107D1)和CYP107X1,也被证明可以催化类固醇羟基化,尽管底物范围有限。在这项研究中,我们从Streptoalloteichus sp. KCCM40925中鉴定出CYP107E41是CYP107家族的一个新成员,对雄甾烷和妊娠激素都具有选择性羟基化活性。使用11种类固醇进行体外筛选,结果显示主要是6β-和16α-羟基化,雄甾酮底物表现出更高的转化效率。在评价的氧化还原体系中,二乙酰氧基碘苯的催化效率最高。分子对接确定了C6-Fe和C16-Fe两种主要的结合取向,其中C6-Fe构型在CNN位姿评价中得分一直较高,表明其是催化首选的结合模式。分子动力学模拟进一步支持了这一点,表明睾酮复合物与位于血红素铁附近的C6保持稳定的构象,与选择性单羟基化一致。相反,诺龙配合物表现出更大的构象灵活性,保持了C6和C7与血红素的接近性,与其更广泛的羟基化谱一致。这些发现表明CYP107E41是一个有希望的区域选择性类固醇羟基化候选者,并为未来的蛋白质工程和氧化还原系统优化提供了机制见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


