Electrodeposition of CuNiS as battery type electrode for supercapacitor applications
IF 4.2
3区 工程技术
Q2 ELECTROCHEMISTRY
引用次数: 0
Abstract
This study focuses on the high-performance, binder-free electrodes on nickel foam by simple cathodic electrodeposition using nickel chloride, copper sulfate, and thiourea solution as aqueous electrolytes. The CuS, NiS, and NiCuS electrodes synthesized by cathodic electrodeposition were characterized by X-ray diffraction, energy dispersive spectra, and scanning electron microscopy. The electrochemical performance of the synthesized positive electrodes was investigated in aqueous potassium hydroxide electrolyte by widespread electroanalytical techniques such as cyclic voltammetry, electrochemical impedance spectroscopy, and galvanostatic charge-discharge. The electrochemical tests revealed that the synthesized electrode materials exhibited significant reversible redox reactions. Among the produced electrodes, CuNiS exhibited high specific capacitance (1026.9 F g−1 at 5 mV s−1; 1338.5 F g−1 at 1 A g−1 current density). The produced NiCuS//activated carbon supercapacitor achieved 890 W kg−1 power and 14.9 Wh kg−1 energy density in the potential range from 0 to 1.40 V. The asymmetric supercapacitor reached 157.8 % of the initial discharge capacity at the end of 5000 charge-discharge cycles. The results of this study indicate that the electrodes produced by the cathodic electrochemical deposition method have excellent potential for use as positive electrodes in supercapacitor applications.
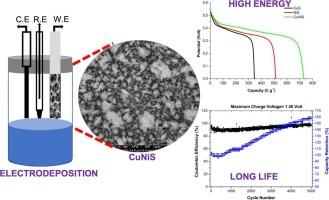
应用于超级电容器电池型电极的CuNiS电沉积研究
本研究以氯化镍、硫酸铜和硫脲溶液为水溶液,采用简单的阴极电沉积方法在泡沫镍上制备高性能无粘结剂电极。采用x射线衍射、能谱和扫描电镜对阴极电沉积法制备的cu、NiS和NiCuS电极进行了表征。利用循环伏安法、电化学阻抗谱、恒流充放电等广泛应用的电分析技术,研究了合成的正极在氢氧化钾水溶液中的电化学性能。电化学测试表明,合成的电极材料表现出明显的可逆氧化还原反应。在所制备的电极中,CuNiS具有较高的比电容(在5 mV s−1时为1026.9 F g−1);1338.5 F g−1在1a g−1电流密度)。在0 ~ 1.40 V电势范围内,NiCuS//活性炭超级电容器的功率为890 W kg−1,能量密度为14.9 Wh kg−1。在5000次充放电循环结束时,非对称超级电容器达到初始放电容量的157.8%。研究结果表明,用阴极电化学沉积法制备的电极在超级电容器中具有良好的正极应用潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochemistry Communications
工程技术-电化学
CiteScore
8.50
自引率
3.70%
发文量
160
审稿时长
1.2 months
期刊介绍:
Electrochemistry Communications is an open access journal providing fast dissemination of short communications, full communications and mini reviews covering the whole field of electrochemistry which merit urgent publication. Short communications are limited to a maximum of 20,000 characters (including spaces) while full communications and mini reviews are limited to 25,000 characters (including spaces). Supplementary information is permitted for full communications and mini reviews but not for short communications. We aim to be the fastest journal in electrochemistry for these types of papers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


