Density functional theory study of adsorption of dissolved gas in transformer oil on Nb -doped SnS2 monolayer
IF 1.8
4区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Based on the density functional theory (DFT), It was found that the adsorption capacity of C2H2, CO and H2 on intrinsic SnS2 was weak, and CH4 on intrinsic SnS2 was strong. Meanwhile, the adsorption energies of these five gas molecules on their surfaces are increased to different degrees. Among them, the adsorption capacity of C2H2 on Nb/SnS2 is stronger, with an adsorption energy of -1.365 eV. From the point of view of the recovery time after adsorption of the gases, at a temperature equal to 498 K, the recovery time of C2H2 gas molecules on Nb/SnS2 is 64.5 s, which makes it a promising gas-sensitive sensor for C2H2. The results show that the replacement of S atoms by Nb atoms in the doping of SnS2 improves the gas adsorption performance of the SnS2 material, and at the same time, its preparation method is close to the reality, and it can be considered to be used as a material for making gas sensors.
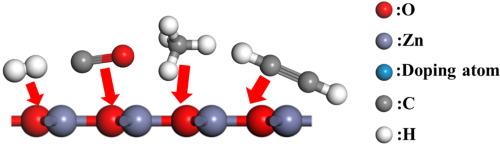
掺铌SnS2单层吸附变压器油中溶解气体的密度泛函理论研究
基于密度泛函理论(DFT),发现C2H2、CO和H2在本征SnS2上的吸附能力较弱,CH4在本征SnS2上的吸附能力较强。同时,这五种气体分子在其表面的吸附能都有不同程度的提高。其中,C2H2对Nb/SnS2的吸附能力较强,吸附能为-1.365 eV。从气体吸附后的回收时间来看,在498 K的温度下,C2H2气体分子在Nb/SnS2上的回收时间为64.5 s,是一种很有前途的C2H2气敏传感器。结果表明,SnS2掺杂中以Nb原子取代S原子提高了SnS2材料的气体吸附性能,同时其制备方法接近实际,可以考虑作为制作气体传感器的材料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Surface Science
化学-物理:凝聚态物理
CiteScore
3.30
自引率
5.30%
发文量
137
审稿时长
25 days
期刊介绍:
Surface Science is devoted to elucidating the fundamental aspects of chemistry and physics occurring at a wide range of surfaces and interfaces and to disseminating this knowledge fast. The journal welcomes a broad spectrum of topics, including but not limited to:
• model systems (e.g. in Ultra High Vacuum) under well-controlled reactive conditions
• nanoscale science and engineering, including manipulation of matter at the atomic/molecular scale and assembly phenomena
• reactivity of surfaces as related to various applied areas including heterogeneous catalysis, chemistry at electrified interfaces, and semiconductors functionalization
• phenomena at interfaces relevant to energy storage and conversion, and fuels production and utilization
• surface reactivity for environmental protection and pollution remediation
• interactions at surfaces of soft matter, including polymers and biomaterials.
Both experimental and theoretical work, including modeling, is within the scope of the journal. Work published in Surface Science reaches a wide readership, from chemistry and physics to biology and materials science and engineering, providing an excellent forum for cross-fertilization of ideas and broad dissemination of scientific discoveries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


