Ido1 or Ido2 deficiency in myeloid-derived cells attenuates TMEV-induced ictogenesis
IF 2.5
4区 医学
Q3 IMMUNOLOGY
引用次数: 0
Abstract
Viral encephalitis is a serious medical condition that causes neuroinflammation, neurodegeneration, cognitive deficits and seizures (ictogenesis), predisposing patients to epilepsy. In a preclinical setting, intracranial infection of C57BL/6 mice with Theiler's murine encephalomyelitis virus (TMEV) is the best-characterized animal model of viral encephalitis resulting in ictogenesis and temporal lobe epilepsy. Macrophages play a critical yet unclear role during encephalomyelitis: macrophage depletion reduces TMEV-induced ictogenesis, whereas prevention of macrophage infiltration into the brain reduces hippocampal damage without changing seizure incidence. Here, we explore the roles of indoleamine-2,3-dioxygenase (Ido) 1 and 2 from myeloid-derived cells (i.e. monocytes, macrophages and monocyte-derived dendritic cells) in the context of TMEV-induced ictogenesis and hippocampal gene expression. Ido1 and Ido2 gene expression is induced by inflammation. IDO1 and IDO2 proteins initiate the kynurenine pathway, by which tryptophan is converted into kynurenine but both proteins have poorly characterized non-enzymatic actions with unknown consequences during encephalomyelitis. In this study, we found that Ido1 and Ido2 deficiencies within myeloid-derived cells reduced ictogenesis without altering hippocampal inflammation-associated gene expression. In vitro infection of peritoneal macrophages demonstrated that genotype does not impact TMEV replication or cytokine expression, suggesting that the direct response of macrophages to infection is not the mechanism for the observed attenuation of ictogenesis in mice with myeloid Ido1 or Ido2 deficiencies.
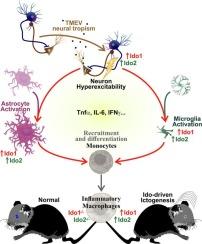
髓源性细胞缺乏Ido1或Ido2可减弱tmev诱导的ictogenesis
病毒性脑炎是一种严重的疾病,可导致神经炎症、神经变性、认知缺陷和癫痫发作(癫痫发生),使患者易患癫痫。在临床前环境中,C57BL/6小鼠颅内感染Theiler小鼠脑脊髓炎病毒(TMEV)是病毒性脑炎导致ictogenesis和颞叶癫痫的最佳表征动物模型。巨噬细胞在脑脊髓炎中发挥着关键但尚不清楚的作用:巨噬细胞耗竭可减少tmev诱导的ictogenesis,而防止巨噬细胞浸润到大脑可减少海马损伤,但不会改变癫痫发作的发生率。在这里,我们探讨了来自髓系细胞(即单核细胞、巨噬细胞和单核细胞来源的树突状细胞)的吲哚胺-2,3-双加氧酶(Ido) 1和2在tmev诱导的ictogenesis和海马基因表达中的作用。炎症诱导Ido1和Ido2基因表达。IDO1和IDO2蛋白启动犬尿氨酸途径,色氨酸通过该途径转化为犬尿氨酸,但这两种蛋白在脑脊髓炎期间的非酶作用特征不明显,后果未知。在这项研究中,我们发现髓源性细胞中的Ido1和Ido2缺乏减少了iclogenesis,而不改变海马炎症相关基因的表达。体外感染的腹腔巨噬细胞表明,基因型不影响TMEV的复制或细胞因子的表达,这表明巨噬细胞对感染的直接反应并不是骨髓Ido1或Ido2缺乏小鼠icogenesis衰减的机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of neuroimmunology
医学-免疫学
CiteScore
6.10
自引率
3.00%
发文量
154
审稿时长
37 days
期刊介绍:
The Journal of Neuroimmunology affords a forum for the publication of works applying immunologic methodology to the furtherance of the neurological sciences. Studies on all branches of the neurosciences, particularly fundamental and applied neurobiology, neurology, neuropathology, neurochemistry, neurovirology, neuroendocrinology, neuromuscular research, neuropharmacology and psychology, which involve either immunologic methodology (e.g. immunocytochemistry) or fundamental immunology (e.g. antibody and lymphocyte assays), are considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


