Noninvasive in vivo tracking of SPIONs-labeled CLDN18.2-targeted CAR-T cells in gastric cancer via magnetic particle imaging
IF 5.9
2区 医学
Q1 MEDICAL LABORATORY TECHNOLOGY
引用次数: 0
Abstract
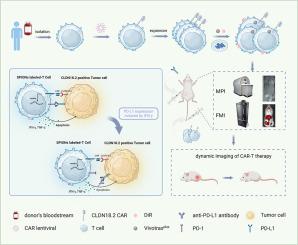
This study aims to employ magnetic particle imaging (MPI) for in vivo tracking and quantitative assessment of targeting capability of CLDN18.2-specific CAR-T cells. CLDN18.2-targeted CAR-T cells were labeled with superparamagnetic iron oxide nanoparticles (SPIONs) for magnetic particle imaging (MPI), and with the near-infrared fluorescent dye DiR for fluorescence molecular imaging (FMI) before infusion. SPIONs-labeled and unlabeled CAR-T cells were administered intravenously to NOD/SCID mice bearing HGC27 xenograft tumors, either independently or in combination with anti-PD-L1 (aPD-L1) antibody (n = 3 for imaging and n = 5 for treatment). The FMI and MPI successfully monitored the dynamic migration and tumor targeting of CAR-T cells towards CLDN18.2-overexpressing tumors. On the fifth day post-infusion, the MPI signal of SPIONs-labeled CAR-T cells was significantly higher in the tumor than that of labeled normal T cells. MPI combined with FMI successfully monitored the targeting of CLDN18.2-specific CAR-T cells in gastric cancer, providing a potential framework for evaluating CAR-T therapy combined with aPD-L1 immunotherapy.
通过磁颗粒成像无创跟踪胃癌中spions标记的cldn18.2靶向CAR-T细胞
本研究旨在利用磁颗粒成像(MPI)技术对cldn18.2特异性CAR-T细胞的体内靶向能力进行跟踪和定量评估。cldn18.2靶向CAR-T细胞在输注前用超顺磁性氧化铁纳米颗粒(SPIONs)标记进行磁颗粒成像(MPI),用近红外荧光染料DiR标记进行荧光分子成像(FMI)。将spons标记和未标记的CAR-T细胞静脉注射给携带HGC27异种移植肿瘤的NOD/SCID小鼠,单独或联合抗pd - l1 (aPD-L1)抗体(n = 3用于成像,n = 5用于治疗)。FMI和MPI成功地监测了CAR-T细胞对过表达cldn18.2的肿瘤的动态迁移和肿瘤靶向。注射后第5天,spions标记的CAR-T细胞在肿瘤中的MPI信号明显高于标记的正常T细胞。MPI联合FMI成功地监测了cldn18.2特异性CAR-T细胞在胃癌中的靶向性,可能指导CAR-T联合aPD-L1免疫治疗的评估。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Translational Research
医学-医学:内科
CiteScore
15.70
自引率
0.00%
发文量
195
审稿时长
14 days
期刊介绍:
Translational Research (formerly The Journal of Laboratory and Clinical Medicine) delivers original investigations in the broad fields of laboratory, clinical, and public health research. Published monthly since 1915, it keeps readers up-to-date on significant biomedical research from all subspecialties of medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


