Localized growth factor delivery from microparticles modulates osteogenic and chondrogenic gene expression in a growth factor-dependent manner in an ex vivo chick embryonic bone model
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Growth factors play a crucial role in regulating various cellular functions, including proliferation and differentiation. Consequently, the biomaterial-based delivery of exogenous growth factors presents a promising strategy in regenerative medicine to manage the healing process and restore tissue function. For effective therapeutic applications, it is essential that these active compounds are precisely targeted to the site of regeneration, with release kinetics that align with the gradual pace of tissue growth. We have developed an ex vivo model utilizing a developing embryonic chick bone, and using PLGA-based microparticles as controlled-release systems, allowing for the investigation of the spatiotemporal effects of growth factor delivery on cell differentiation and tissue formation. Our findings demonstrate that BMP2 and FGF2 can significantly alter cell morphology and zonally pattern collagen deposition within the model, but only when the growth factor presentation rate is carefully regulated. Furthermore, the growth factor-dependent responses observed underscore the potential of this model to explore interactions between cells and the growth factors released from biomaterials in an approach which can be applied to bone tissue engineering.
Statement of significance
Current biomaterial-based strategies for bone tissue engineering face critical limitations in mimicking the spatial and temporal dynamics of native tissue development. This study introduces an innovative ex vivo embryonic chick bone model to evaluate localized, sustained growth factor delivery using PLGA microparticles. By precisely controlling the release of BMP2 and FGF2, the research demonstrates growth factor-specific modulation of osteogenic and chondrogenic gene expression and matrix deposition, outcomes that traditional in vitro models fail to capture. This physiologically relevant platform bridges a critical gap between basic in vitro assays and complex in vivo models, offering a powerful, low-cost tool for preclinical screening of regenerative therapies, and advancing the rational design of next-generation bone healing strategies.
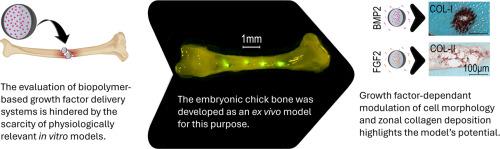
在离体鸡胚胎骨模型中,微颗粒局部递送生长因子以生长因子依赖的方式调节成骨和软骨基因的表达。
生长因子在调节多种细胞功能,包括增殖和分化中起着至关重要的作用。因此,基于生物材料的外源性生长因子递送在再生医学中是一种很有前途的策略,可以管理愈合过程和恢复组织功能。为了有效的治疗应用,这些活性化合物必须精确地靶向再生部位,释放动力学与组织生长的逐渐速度一致。我们开发了一个体外模型,利用发育中的胚胎鸡骨,并使用基于plga的微颗粒作为控释系统,允许研究生长因子递送对细胞分化和组织形成的时空效应。我们的研究结果表明,BMP2和FGF2可以显著改变细胞形态和胶原沉积的带状模式,但只有在生长因子呈递率被仔细调节的情况下。此外,观察到的生长因子依赖性反应强调了该模型在探索细胞与生物材料释放的生长因子之间相互作用方面的潜力,该方法可应用于骨组织工程。意义声明:目前基于生物材料的骨组织工程策略在模拟天然组织发育的时空动态方面面临着严重的限制。本研究介绍了一种创新的离体鸡胚胎骨模型,以评估PLGA微粒局部持续的生长因子输送。通过精确控制BMP2和FGF2的释放,该研究证明了生长因子对成骨和软骨基因表达和基质沉积的特异性调节,这是传统体外模型无法捕获的结果。这个生理学相关的平台在基本的体外实验和复杂的体内模型之间架起了桥梁,为再生疗法的临床前筛选提供了一个强大的、低成本的工具,并推动了下一代骨愈合策略的合理设计。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


