Unraveling the power of TOMM40: Driving PHB1-mediated mtDNA release and mitophagy to fuel breast cancer progression
IF 2.2
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. General subjects
Pub Date : 2025-08-13
DOI:10.1016/j.bbagen.2025.130851
引用次数: 0
Abstract
Background
In breast cancer (BRCA), mitophagy is essential for the survival and metastasis of cancer cells. However, the interaction between translocase of the outer mitochondrial membrane 40 (TOMM40) and prohibitin 1 (PHB1) in regulating mitophagy in BRCA remains poorly understood.
Methods
Based on bioinformatics analysis, the interaction between PHB1 and key mitophagy regulators in BRCA was explored. The effects of mitochondrial division inhibitor-1 (Mdivi-1) and Fluorizoline on mitophagy, cell viability, and sphere formation ability in MDA-MB-231 cells were assessed. In the cell model activated by carbonyl cyanide m-chlorophenylhydrazone (CCCP) to induce mitophagy, the effects of TOMM40 on cell viability, sphere formation ability, mitochondrial membrane potential, reactive oxygen species (ROS) levels, mitochondrial DNA (mtDNA) release, and PHB1 regulation were analyzed. In vivo, the impact of TOMM40 knockdown on tumor progression and mitophagy was also evaluated.
Results
PHB1 interacted with TOMM40. Mdivi-1 or Fluorizoline treatment inhibited mitophagy, and significantly reduced BRCA cell viability and sphere formation. CCCP treatment induced mitophagy, increased mtDNA release and PHB1 levels, decreased mitochondrial membrane potential and ROS, and promoted cell viability and sphere formation ability, which were all reversed by TOMM40 knockdown. Additionally, TOMM40 knockdown led to decreased PHB1 levels and increased ROS accumulation in tumor tissue, thus repressing tumor progression.
Conclusion
This study identifies TOMM40 as a key regulator that enhances PHB1-mediated mtDNA release and induces mitophagy in BRCA cells, thus promoting breast cancer progression.
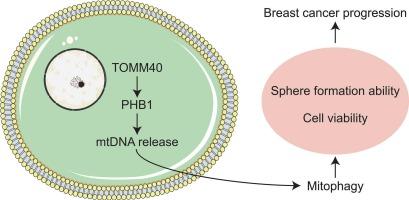
揭示TOMM40的力量:驱动phb1介导的mtDNA释放和线粒体自噬促进乳腺癌进展
背景:在乳腺癌(BRCA)中,线粒体自噬对癌细胞的生存和转移至关重要。然而,线粒体外膜转座酶40 (TOMM40)和禁止蛋白1 (PHB1)在调节BRCA线粒体自噬中的相互作用仍然知之甚少。方法:基于生物信息学分析,探讨PHB1与BRCA中关键丝裂调节因子之间的相互作用。观察线粒体分裂抑制剂-1 (Mdivi-1)和氟唑啉对MDA-MB-231细胞线粒体自噬、细胞活力和成球能力的影响。在羰基氰化物间氯苯腙(CCCP)诱导线粒体自噬的细胞模型中,分析了TOMM40对细胞活力、成球能力、线粒体膜电位、活性氧(ROS)水平、线粒体DNA (mtDNA)释放和PHB1调控的影响。在体内,我们还评估了TOMM40基因敲低对肿瘤进展和线粒体自噬的影响。结果:PHB1与TOMM40相互作用。Mdivi-1或氟唑啉处理抑制了有丝分裂,显著降低了BRCA细胞活力和球的形成。CCCP处理诱导线粒体自噬,增加mtDNA释放和PHB1水平,降低线粒体膜电位和ROS,提高细胞活力和成球能力,这些都被TOMM40敲除逆转。此外,TOMM40敲低导致肿瘤组织中PHB1水平降低,ROS积累增加,从而抑制肿瘤进展。结论:本研究确定TOMM40是促进phb1介导的BRCA细胞mtDNA释放,诱导线粒体自噬,从而促进乳腺癌进展的关键调控因子。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochimica et biophysica acta. General subjects
生物-生化与分子生物学
CiteScore
6.40
自引率
0.00%
发文量
139
审稿时长
30 days
期刊介绍:
BBA General Subjects accepts for submission either original, hypothesis-driven studies or reviews covering subjects in biochemistry and biophysics that are considered to have general interest for a wide audience. Manuscripts with interdisciplinary approaches are especially encouraged.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


