A sustainable process for vanadium recovery from stone coal: efficient enrichment via alkali melting–carbothermal reduction
IF 5
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
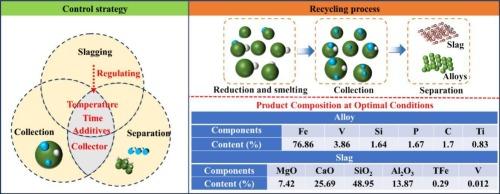
With the continuous growth of vanadium market demand, it has become increasingly urgent to promote research on sustainable and efficient vanadium resource utilization. Stone coal vanadium ore has remained underutilized due to its low grade and complex composition. This study proposes a calcium oxide (CaO)-assisted fusion and carbothermal reduction process for the efficient release and enrichment of vanadium from stone coal. The experiment systematically investigated the effects of reduction temperature, slag composition, reducing agent dosage, and smelting time on vanadium recovery efficiency. Calculation results indicate that vanadium pentoxide (V2O5) begins to decompose at 288 °C, and reduction to elemental vanadium (V) requires a temperature of approximately 1600 °C. Below 1468 °C, iron and vanadium form a continuous solid solution. Above 1538 °C, molten iron exhibits a strong affinity for vanadium, acting as a collector. Under optimized conditions (1600 °C for 2 h, 35 % calcium oxide, 5 % carbon, and 9 % ferric oxide), the vanadium enrichment ratio reaches 97.84 %. The resultant vanadium-iron alloy contains 4.09 % V and 78.35 % total iron (TFe). The chemical composition of the tailings closely resembles that of rock wool raw materials, indicating significant potential for resource utilization. The stability and industrial application prospect of the process were further confirmed by pilot test. This study serves as a technical reference for the sustainable and efficient development of stone coal vanadium ore, as well as the high-value utilization of vanadium resources.
石煤中钒的可持续回收工艺:碱熔融-碳热还原高效富集
随着钒市场需求的不断增长,推动钒资源可持续高效利用的研究日益迫切。石煤钒矿石品位低、成分复杂,一直未得到充分利用。本研究提出了一种氧化钙(CaO)辅助熔融碳热还原工艺,用于石煤中钒的高效释放和富集。实验系统考察了还原温度、炉渣组成、还原剂用量、冶炼时间对钒回收率的影响。计算结果表明,五氧化二钒(V2O5)在288℃时开始分解,还原为单质钒(V)需要1600℃左右的温度。在1468℃以下,铁和钒形成连续的固溶体。在1538°C以上,铁液对钒表现出很强的亲和力,起到捕收剂的作用。在1600℃、氧化钙35%、碳5%、氧化铁9%的条件下,钒的富集率可达97.84%。所得钒铁合金含4.09% V和78.35%总铁(TFe)。尾矿的化学成分与岩棉原料的化学成分非常接近,具有很大的资源利用潜力。中试进一步证实了该工艺的稳定性和工业应用前景。为石煤钒矿的可持续高效开发和钒资源的高价值利用提供技术参考。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Minerals Engineering
工程技术-工程:化工
CiteScore
8.70
自引率
18.80%
发文量
519
审稿时长
81 days
期刊介绍:
The purpose of the journal is to provide for the rapid publication of topical papers featuring the latest developments in the allied fields of mineral processing and extractive metallurgy. Its wide ranging coverage of research and practical (operating) topics includes physical separation methods, such as comminution, flotation concentration and dewatering, chemical methods such as bio-, hydro-, and electro-metallurgy, analytical techniques, process control, simulation and instrumentation, and mineralogical aspects of processing. Environmental issues, particularly those pertaining to sustainable development, will also be strongly covered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


