Impact of steam sterilization on the rheological characteristics, printability, and bioactivity of nanocellulose/alginate hydrogels for 3D bioprinting
IF 6.5
Q1 CHEMISTRY, APPLIED
Carbohydrate Polymer Technologies and Applications
Pub Date : 2025-08-12
DOI:10.1016/j.carpta.2025.100970
引用次数: 0
Abstract
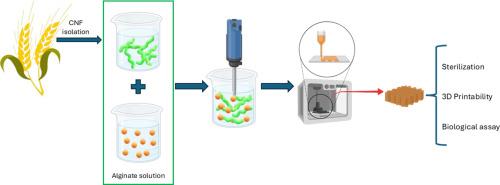
Effective sterilization of bioinks is essential for the clinical translation of 3D bioprinting, though it can compromise the physical and biological properties of natural hydrogels. This study examines the impact of steam sterilization (121 °C, 15 min) on nanocellulose (CNF)/alginate hydrogels with alginate concentrations of 1 %, 1.5 %, and 3 % (w/v), focusing on rheology, printability, and biocompatibility. Hydrogels with 1.5 % and 3 % alginate retained their viscoelastic properties after sterilization, with storage modulus (G′) values above 1000 Pa. Printability analysis showed high structural fidelity, with pore factors of 0.75–0.88 and integrity factors over 0.9. In contrast, the 1 % formulation displayed poor crosslinking and structural instability. Human bone marrow-derived mesenchymal stem cells (hB MMSCs) showed over 90 % viability in all sterilized hydrogels. The 3 % alginate formulation supported uniform cell distribution and scaffold integrity over 14 days, despite slightly reduced cell proliferation. Its superior mechanical strength and dimensional stability make it the most suitable for applications requiring robust and precise scaffolds. These findings highlight the importance of optimizing alginate concentration, particularly at 3 % w/v, to ensure a balance between sterilization resilience, printability, and biocompatibility. This work provides a validated strategy for developing safe, effective, and ready-to-use bioinks for tissue engineering and regenerative medicine.
蒸汽灭菌对3D生物打印纳米纤维素/海藻酸盐水凝胶流变特性、可打印性和生物活性的影响
生物墨水的有效灭菌对于3D生物打印的临床翻译至关重要,尽管它可能会损害天然水凝胶的物理和生物特性。本研究考察了蒸汽灭菌(121°C, 15分钟)对海藻酸盐浓度分别为1%、1.5%和3% (w/v)的纳米纤维素(CNF)/海藻酸盐水凝胶的影响,重点研究了流变性、可打印性和生物相容性。含有1.5%和3%海藻酸盐的水凝胶在灭菌后仍保持粘弹性,其储存模量(G′)值在1000 Pa以上。打印适性分析显示,结构保真度高,孔隙系数在0.75 ~ 0.88之间,完整性系数在0.9以上。相比之下,1%的配方表现出较差的交联和结构不稳定性。人骨髓间充质干细胞(hB MMSCs)在所有无菌水凝胶中显示超过90%的存活率。3%海藻酸盐配方在14天内支持均匀的细胞分布和支架完整性,尽管细胞增殖略有减少。其优异的机械强度和尺寸稳定性使其最适合需要坚固和精密支架的应用。这些发现强调了优化海藻酸盐浓度的重要性,特别是在3% w/v时,以确保灭菌弹性,印刷性和生物相容性之间的平衡。这项工作为组织工程和再生医学开发安全、有效和即用型生物墨水提供了一种有效的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


