Biocontrol potential and mechanism of Bacillus velezensis YMG-03 for sesame diseases
IF 3.3
3区 农林科学
Q2 PLANT SCIENCES
引用次数: 0
Abstract
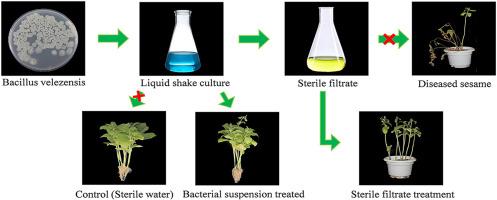
Sesame, a globally important oilseed crop, faces significant threats from fungal diseases, leading to substantial yield and quality losses. Utilizing antagonistic bacteria for biological control offers a sustainable and environmentally friendly alternative to chemical fungicides in sustainable agriculture. This study investigated the control effects and mechanisms of the Bacillus velezensis strain YMG-03 against sesame diseases. In dual-culture comparison assays, YMG-03 broadly inhibited eight major sesame pathogens, achieving 83.73% suppression against Macrophomina phaseolina, causing sesame stem blight. Scanning electron microscopy revealed severe morphological disruption of M. phaseolina hyphae following a dual-culture comparison assay with strain YMG-03. Pot experiments demonstrated that YMG-03 significantly suppressed stem blight disease and enhanced sesame growth. Genome analysis of strain YMG-03 revealed a 3.93 Mb circular chromosome containing 12 secondary metabolite biosynthetic gene clusters (e.g., surfactin, fengycin, bacillibactin) and genes encoding hydrolytic enzymes (e.g., chitinase, cellulase), indicating its genetic potential for antagonistic metabolite and hydrolases production. Untargeted metabolomics identified 1413 up-regulated metabolites, among which lipids (e.g., liquiritigenin, jasmonic acid, capsaicin), organic acids (e.g., 6-aminopenicillanic acid, piperic acid, coumaric acid), and key precursors for biocontrol metabolites (3-hydroxy fatty acids, N-acetylglucosamine, L-leucine) significantly accumulated. KEGG enrichment analysis revealed their association with fatty acid metabolism, polyketide/terpenoid biosynthesis, and amino sugar metabolism, consistent with the predicted antagonistic functions from genomic data. These findings systematically elucidate the dual role of B. velezensis YMG-03 in synergistically promoting sesame growth and controlling stem blight, and provide both strain resources and theoretical foundations for applying B. velezensis in biological control.
麦氏芽孢杆菌YMG-03对芝麻病的生物防治潜力及作用机制
芝麻是一种全球重要的油料作物,面临真菌病害的严重威胁,导致产量和质量大幅下降。利用拮抗菌进行生物防治为可持续农业提供了一种可持续的、环境友好的化学杀菌剂替代品。本试验研究了velezensis芽孢杆菌YMG-03对芝麻病害的防治作用及其机制。在双培养对比试验中,YMG-03对8种主要芝麻病原菌均有广泛抑制作用,对引起芝麻茎枯病的菜籽病(Macrophomina phaseolina)的抑制率达到83.73%。扫描电镜显示,在与菌株YMG-03的双重培养比较中,phaseolina菌丝严重形态破坏。盆栽试验表明,YMG-03能显著抑制干枯病,促进芝麻生长。菌株YMG-03的基因组分析显示,其3.93 Mb的环状染色体包含12个次级代谢物生物合成基因簇(如surfactin、fengycin、bacillibactin)和编码水解酶的基因簇(如几丁质酶、纤维素酶),表明其具有拮抗代谢物和水解酶生产的遗传潜力。非靶向代谢组学鉴定出1413种上调代谢物,其中脂质(如利尿原素、茉莉酸、辣椒素)、有机酸(如6-氨基青霉酸、胡椒酸、香豆酸)和生物防治代谢物关键前体(3-羟基脂肪酸、n -乙酰氨基葡萄糖胺、l -亮氨酸)显著积累。KEGG富集分析显示它们与脂肪酸代谢、聚酮/萜类生物合成和氨基糖代谢有关,与基因组数据预测的拮抗功能一致。这些研究结果系统地阐明了白僵菌YMG-03在促进芝麻生长和防治茎枯病中的双重作用,为白僵菌的生物防治提供了菌株资源和理论基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
4.30
自引率
7.40%
发文量
130
审稿时长
38 days
期刊介绍:
Physiological and Molecular Plant Pathology provides an International forum for original research papers, reviews, and commentaries on all aspects of the molecular biology, biochemistry, physiology, histology and cytology, genetics and evolution of plant-microbe interactions.
Papers on all kinds of infective pathogen, including viruses, prokaryotes, fungi, and nematodes, as well as mutualistic organisms such as Rhizobium and mycorrhyzal fungi, are acceptable as long as they have a bearing on the interaction between pathogen and plant.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


