WB-assisted activation of periodate by Fe(III) for decontamination: Periodate concentration-dependent mechanistic shift
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
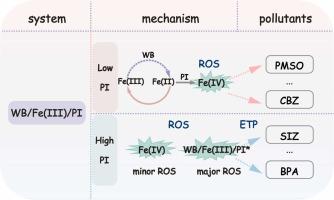
The iron-activated periodate (PI) process may be limited by the sluggish kinetics of Fe(II) regeneration and Fe(III) accumulation. Herein, tungsten boride (WB), serving as a co-catalyst, can effectively overcome the inherent drawback of oxidation reactions. The WB/Fe(III)/PI system exhibited different removal efficiencies toward sulfisoxazole (SIZ) under varying PI concentrations. Based on the qualitative and semiquantitative tests, the generation of reactive species changes with various PI concentrations. Specifically, at low PI concentration, Fe(IV), generated via Fe(II)-mediated PI activation following WB-driven Fe(III) reduction, was the primary reactive species responsible for rapid SIZ removal. However, at high PI concentrations, the limited generation of Fe(IV) contributes only marginally to SIZ removal. In situ Raman characterization and electrochemical tests revealed that at high PI concentration, WB could react with PI and Fe(III) to form high-potential metastable intermediates (WB-Fe(III)-PI*), facilitating direct electron transfer pathway (ETP) from the SIZ to PI. Density functional theory (DFT) calculations further confirmed the interactions among WB, Fe(III), and PI. Besides, at low PI concentrations, the WB/Fe(III)/PI system selectively and rapidly oxidized oxygen-transfer-prone pollutants, while at high PI concentrations, it favored sustained oxidation of electron-rich pollutants. Additionally, WB exhibits excellent reusability during cyclic use. This comprehensive investigation deepens insight into PI-based co-catalytic process and offers an efficient strategy for selecting appropriate oxidant concentrations for wastewater treatment.
wb辅助铁(III)激活高碘酸盐净化:高碘酸盐浓度依赖的机制转移
铁活化高碘酸盐(PI)过程可能受到铁(II)再生和铁(III)积累缓慢动力学的限制。其中,硼化钨(WB)作为助催化剂,可以有效地克服氧化反应固有的缺点。在不同的PI浓度下,WB/Fe(III)/PI体系对磺胺恶唑(SIZ)的去除率不同。定性和半定量实验表明,不同浓度的PI会改变反应物质的生成。具体来说,在低PI浓度下,通过Fe(II)介导的PI激活在wb驱动的Fe(III)还原后产生的Fe(IV)是负责快速去除SIZ的主要活性物质。然而,在高PI浓度下,有限生成的Fe(IV)对SIZ去除的贡献很小。原位拉曼表征和电化学测试表明,在高PI浓度下,WB可以与PI和Fe(III)反应形成高电位亚稳中间体(WB-Fe(III)-PI*),促进了SIZ到PI的直接电子转移途径(ETP)。密度泛函理论(DFT)计算进一步证实了WB、Fe(III)和PI之间的相互作用。此外,在低PI浓度下,WB/Fe(III)/PI体系选择性地快速氧化易氧转移的污染物,而在高PI浓度下,它有利于富电子污染物的持续氧化。此外,WB在循环使用过程中表现出出色的可重用性。这项全面的研究加深了对基于pi的共催化过程的了解,并为选择适当的氧化剂浓度进行废水处理提供了有效的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


