Adsorption of PFCAs using polyethyleneimine modified Biochar: Role of chain length and effects of water matrices
IF 8.1
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
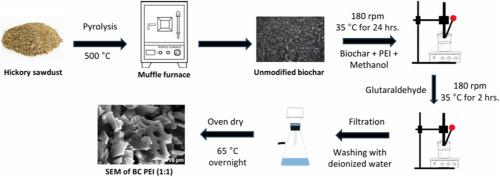
Perfluorocarboxylic acids (PFCAs) are emerging organic pollutants posing a threat to human health and the environment. This study investigates the efficacy of polyethyleneimine-modified biochar (BC-PEI) as an adsorbent for removing PFCAs from a mixed solute system, focusing on competitive adsorption among PFCAs with varying chain lengths. It includes perfluorooctanoic acid (PFOA), perfluorohexanoic acid (PFHxA), hexafluoropropylene-oxide-dimer-acid (GenX), and perfluorobutanoic acid (PFBA). BC-PEI (1:1) (wPEI/wBC = 1) exhibited the highest adsorption capacities for PFOA, PFHxA, GenX, and PFBA at 1.302, 0.850, 0.711, and 0.397 mmol/g, respectively. It follows the Sips isotherm (Langmuir-Freundlich isotherm) model, which becomes Langmuir at high concentration and Freundlich at low concentration. Surface functional groups, as well as electrostatic and hydrophobic interactions, influenced the adsorption mechanism. Long-chain PFCAs demonstrated higher adsorption capacities due to stronger hydrophobic interactions, while short-chain PFCAs were primarily adsorbed via electrostatic interactions. Kinetics data were best described by the pseudo-second-order (PSO) model, with surface adsorption and minor micropore contributions governing the process. The presence of humic acid reduced the adsorption capacity by competing for adsorption sites. The background ions in the aqueous matrix further diminished capacity due to double-layer compression. Fourier-transform infrared spectroscopy (FT-IR) and X-ray photoelectron spectroscopy (XPS) confirmed PFCAs adsorption onto BC-PEI. These findings underscore the potential of BC-PEI as a promising adsorbent for PFCA remediation in wastewater systems, highlighting its engineering applications.
聚乙烯亚胺改性生物炭对PFCAs的吸附:链长和水基质的作用
全氟羧酸是对人类健康和环境构成威胁的新型有机污染物。本研究考察了聚乙烯亚胺改性生物炭(BC-PEI)作为吸附剂从混合溶质体系中去除PFCAs的效果,重点研究了不同链长PFCAs之间的竞争吸附。它包括全氟辛酸(PFOA)、全氟己酸(PFHxA)、六氟丙烯二聚酸(GenX)和全氟丁酸(PFBA)。BC-PEI (1:1) (wPEI/wBC = 1)对PFOA、PFHxA、GenX和PFBA的吸附量最高,分别为1.302、0.850、0.711和0.397 mmol/g。它遵循Sips等温线(Langmuir-Freundlich等温线)模式,高浓度时为Langmuir,低浓度时为Freundlich。表面官能团以及静电和疏水相互作用影响了吸附机理。由于较强的疏水相互作用,长链PFCAs表现出较高的吸附能力,而短链PFCAs主要通过静电相互作用吸附。动力学数据最好用伪二阶(PSO)模型来描述,表面吸附和微孔的贡献控制了这一过程。腐植酸的存在通过竞争吸附位点降低了吸附能力。由于双层压缩,水基中的背景离子进一步降低了容量。傅里叶变换红外光谱(FT-IR)和x射线光电子能谱(XPS)证实了PFCAs在BC-PEI上的吸附。这些发现强调了BC-PEI作为污水系统中PFCA修复的吸附剂的潜力,强调了其工程应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemosphere
环境科学-环境科学
CiteScore
15.80
自引率
8.00%
发文量
4975
审稿时长
3.4 months
期刊介绍:
Chemosphere, being an international multidisciplinary journal, is dedicated to publishing original communications and review articles on chemicals in the environment. The scope covers a wide range of topics, including the identification, quantification, behavior, fate, toxicology, treatment, and remediation of chemicals in the bio-, hydro-, litho-, and atmosphere, ensuring the broad dissemination of research in this field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


