Ascovirus suppresses feeding and growth in Spodoptera litura larvae by targeting the neuropeptide F
IF 1.3
3区 农林科学
Q3 ENTOMOLOGY
引用次数: 0
Abstract
Ascoviruses, a family of large, double-stranded circular DNA viruses, exhibit high host specificity and pathogenicity, suggesting their significant potential in biocontrol. A hallmark characteristic of ascovirus infection in larvae is reduced feeding and retarded growth. However, the mechanisms by which ascoviruses regulate these effects remain largely unknown. Given their crucial role in regulating larval feeding, insect neuropeptides have attracted our attention in the context of ascovirus infection. During HvAV-3h infection in S. litura, the expression levels of neuropeptide F (NPF), including NPF1 and NPF2, which are integral to feeding regulation, were significantly reduced. HvAV-3h infection impaired NPF regulation in larvae, leading to reduced food intake and larval weight gain across different physiological states. Concurrently, significant up-regulation of the NPF receptor (NPFR) was observed in the head tissue. The observed dysregulation of the NPF/NPFR signaling pathway was associated with elevated juvenile hormone (JH) titers. In contrast, the expression levels of short neuropeptide F (sNPF) and the molting hormone ecdysone remained unchanged. Moreover, histopathological analysis of the midgut revealed no epithelial cell damage. Furthermore, RNA interference of NPF1 or NPF2 significantly increased the expression of NPFR and juvenile hormone acid O-methyltransferase (JHAMT), and tended to further reduce food intake and weight gain, which consequently increased the mortality during HvAV-3h infection. HvAV-3h infection disrupts the NPF/NPFR signaling pathway in S. litura larvae, subsequently elevating JH titers, ultimately leading to reduced food intake and larval weight gain. This study enhances our understanding of the interaction between HvAV-3h and its host, and provides a theoretical basis for the development of innovative pest management strategies.
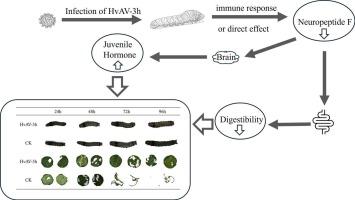
Ascovirus以神经肽F为靶点抑制斜纹夜蛾幼虫的摄食和生长
子囊病毒是一类大型双链环状DNA病毒,具有较高的宿主特异性和致病性,在生物防治方面具有重要潜力。子囊病毒感染幼虫的一个显著特征是摄食减少和生长迟缓。然而,ascov调节这些作用的机制在很大程度上仍然未知。鉴于其在调节幼虫摄食中的关键作用,昆虫神经肽在子囊病毒感染的背景下引起了我们的注意。HvAV-3h感染斜纹海棠时,神经肽F (NPF)的表达水平显著降低,包括NPF1和NPF2, NPF1和NPF2是摄食调节不可或缺的组成部分。HvAV-3h感染破坏了幼虫对NPF的调节,导致不同生理状态下的摄食减少和幼虫体重增加。同时,在头部组织中观察到NPF受体(NPFR)显著上调。观察到的NPF/NPFR信号通路失调与幼体激素(JH)滴度升高有关。短神经肽F (sNPF)和蜕皮激素(ecdysone)的表达水平保持不变。此外,中肠的组织病理学分析显示没有上皮细胞损伤。此外,NPF1或NPF2的RNA干扰显著增加了NPFR和幼年激素酸o -甲基转移酶(JHAMT)的表达,并有进一步减少食物摄入量和体重增加的趋势,从而增加了HvAV-3h感染期间的死亡率。HvAV-3h感染破坏斜纹夜蛾幼虫的NPF/NPFR信号通路,随后升高JH滴度,最终导致摄食减少和幼虫体重增加。本研究增强了我们对HvAV-3h与其宿主相互作用的认识,并为创新害虫防治策略的制定提供了理论基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Asia-pacific Entomology
Agricultural and Biological Sciences-Insect Science
CiteScore
2.70
自引率
6.70%
发文量
152
审稿时长
69 days
期刊介绍:
The journal publishes original research papers, review articles and short communications in the basic and applied area concerning insects, mites or other arthropods and nematodes of economic importance in agriculture, forestry, industry, human and animal health, and natural resource and environment management, and is the official journal of the Korean Society of Applied Entomology and the Taiwan Entomological Society.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


