Phenacyl pyridinium salt-benzoxazine: a photocation generator for benzoxazine ring opening polymerization
IF 6.3
2区 化学
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
This study reports a novel photoactive benzoxazine monomer containing a phenacyl pyridinium salt moiety (P-Pyr_PA-SbF6), which is designed to initiate cationic ring-opening of benzoxazines upon light exposure. For the purpose, the synthesis of the precursor pyridine-functionalized benzoxazine (P-Pyr) and its subsequent conversion to phenacyl pyridinium salts were successfully achieved. The chemical structures of benzoxazines were confirmed by spectroscopic analyses (1H NMR, 13C NMR, FTIR). Photophysical studies demonstrated the photoactivity of P-Pyr_PA-SbF6, evidenced by UV photobleaching and NMR analysis. Differential Scanning Calorimetry (DSC) showed that UV irradiation (360 nm) significantly reduced the self-curing temperature and enthalpy of P-Pyr_PA-SbF6 itself. Furthermore, P-Pyr_PA-SbF6 exhibited effective thermal and photo-assisted catalytic activity for polymerizing a conventional monofunctional benzoxazine monomer (P-a). Light pre-treatment of P-a blends containing P-Pyr_PA-SbF6 substantially lowered the curing onset temperatures (e.g., from 222 °C for neat P-a to 146 °C with 10 mol% salt) and reduced the activation energy (Ea) for P-a polymerization. These findings reveal P-Pyr_PA-SbF6 as a promising system for achieving controlled, lower-energy curing of polybenzoxazines via light induction.
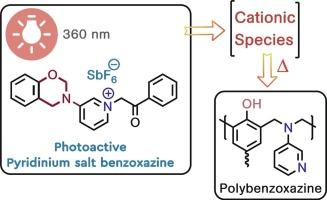
苯并恶嗪:苯并恶嗪开环聚合的光位发生器
本研究报道了一种新型光活性苯并恶嗪单体,该单体含有苯并恶嗪基吡啶盐部分(P-Pyr_PA-SbF6),其设计用于在光照射下引发苯并恶嗪的阳离子开环。为此,成功地合成了前驱体吡啶功能化苯并恶嗪(P-Pyr),并将其转化为苯那基吡啶盐。通过1H NMR、13C NMR、FTIR等光谱分析证实了苯并恶嗪类化合物的化学结构。光物理研究证实了P-Pyr_PA-SbF6的光活性,并通过紫外光漂白和核磁共振分析得到了证实。差示扫描量热法(DSC)表明,360 nm的紫外辐照显著降低了P-Pyr_PA-SbF6本身的自固化温度和自固化焓。此外,P-Pyr_PA-SbF6对常规单官能苯并恶嗪单体(P-a)的聚合表现出有效的热催化和光辅助催化活性。含有P-Pyr_PA-SbF6的P-a共混物的轻预处理大大降低了固化开始温度(例如,纯P-a从222°C降至10 mol%盐的146°C),降低了P-a聚合的活化能(Ea)。这些发现表明P-Pyr_PA-SbF6是一种很有前途的系统,可以通过光诱导实现可控的、低能量的聚苯并恶嗪固化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

European Polymer Journal
化学-高分子科学
CiteScore
9.90
自引率
10.00%
发文量
691
审稿时长
23 days
期刊介绍:
European Polymer Journal is dedicated to publishing work on fundamental and applied polymer chemistry and macromolecular materials. The journal covers all aspects of polymer synthesis, including polymerization mechanisms and chemical functional transformations, with a focus on novel polymers and the relationships between molecular structure and polymer properties. In addition, we welcome submissions on bio-based or renewable polymers, stimuli-responsive systems and polymer bio-hybrids. European Polymer Journal also publishes research on the biomedical application of polymers, including drug delivery and regenerative medicine. The main scope is covered but not limited to the following core research areas:
Polymer synthesis and functionalization
• Novel synthetic routes for polymerization, functional modification, controlled/living polymerization and precision polymers.
Stimuli-responsive polymers
• Including shape memory and self-healing polymers.
Supramolecular polymers and self-assembly
• Molecular recognition and higher order polymer structures.
Renewable and sustainable polymers
• Bio-based, biodegradable and anti-microbial polymers and polymeric bio-nanocomposites.
Polymers at interfaces and surfaces
• Chemistry and engineering of surfaces with biological relevance, including patterning, antifouling polymers and polymers for membrane applications.
Biomedical applications and nanomedicine
• Polymers for regenerative medicine, drug delivery molecular release and gene therapy
The scope of European Polymer Journal no longer includes Polymer Physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


