Design and characterization of BPAC-SA beads synergistic phytoremediation with Eichhornia crassipes for enhanced adsorption of Ni2+ ions from industrial effluents: IoT real-time monitoring
Q1 Environmental Science
引用次数: 0
Abstract
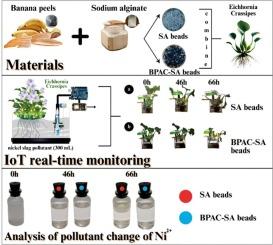
Here, we demonstrate the effective use of sodium alginate (SA) and banana peel-activated carbon (BPAC) combined Eichhornia crassipes for phytoremediation as sustainable adsorbents for Ni2+ ion removal from nickel industry effluents. SA beads' adsorption capacity and removal efficiency exhibited 1357 mg/g and 90.5 %, while BPAC-SA composite beads achieved 1393 mg/g and 92.9 %. Notable bead expansion post-adsorption confirmed strong pollutant interaction, particularly in BPAC-SA beads. Structural characterization via XRD revealed the slight transition from amorphous to crystalline phases, confirming the successful incorporation of BPAC into the SA matrix through ionotropic gelation methods. The BPAC-SA beads displayed a rough, porous morphology with enhanced surface area and bonding potential. At the same time, FTIR spectra showed functional group shifts—especially in ![]() OH,
OH, ![]() CH, carbonyl, and carboxylate bands indicating strong interactions and new bond formations. Real-time monitoring using Internet of Things devices successfully implemented and validated the adsorption kinetics and process efficiency.
CH, carbonyl, and carboxylate bands indicating strong interactions and new bond formations. Real-time monitoring using Internet of Things devices successfully implemented and validated the adsorption kinetics and process efficiency.
BPAC-SA微球协同植物修复对工业废水中Ni2+离子吸附的设计与表征:物联网实时监测
在这里,我们展示了海藻酸钠(SA)和香蕉皮活性炭(BPAC)组合的植物修复方法作为镍工业废水中Ni2+离子的可持续吸附剂的有效使用。SA珠的吸附量和去除率分别为1357 mg/g和90.5%,BPAC-SA复合珠的吸附量和去除率分别为1393 mg/g和92.9%。吸附后明显的颗粒膨胀证实了强的污染物相互作用,特别是在BPAC-SA颗粒中。通过x射线衍射(XRD)进行的结构表征显示,BPAC从非晶相到结晶相有轻微的转变,证实了BPAC通过离子化胶凝方法成功地结合到SA基体中。BPAC-SA珠具有粗糙的多孔形态,具有增强的表面积和键合电位。同时,FTIR光谱显示官能团移位,特别是在OH, CH,羰基和羧酸盐波段,表明强相互作用和新键形成。利用物联网设备进行实时监测,成功实现并验证了吸附动力学和工艺效率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioresource Technology Reports
Environmental Science-Environmental Engineering
CiteScore
7.20
自引率
0.00%
发文量
390
审稿时长
28 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


