RNA Pol II inhibition activates cell death independently from the loss of transcription
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
RNA Pol II-mediated transcription is essential for eukaryotic life. Although loss of transcription is thought to be universally lethal, the associated mechanisms promoting cell death are not yet known. Here, we show that death following the loss of RNA Pol II activity does not result from dysregulated gene expression. Instead, it occurs in response to loss of the hypophosphorylated form of Rbp1 (also called RNA Pol IIA). Loss of RNA Pol IIA exclusively activates apoptosis, and expression of a transcriptionally inactive version of Rpb1 rescues cell viability. Using functional genomics, we identify the mechanisms driving lethality following the loss of RNA Pol IIA, which we call the Pol II degradation-dependent apoptotic response (PDAR). Using the genetic dependencies of PDAR, we identify clinically used drugs that owe their lethality to a PDAR-dependent mechanism. Our findings unveil an apoptotic signaling response that contributes to the efficacy of a wide array of anti-cancer therapies.
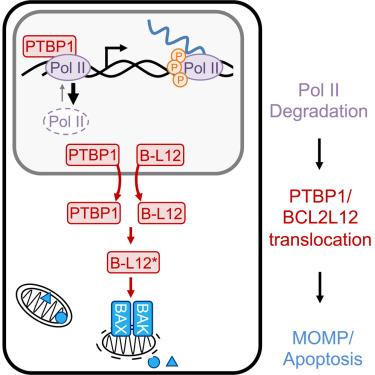
RNA Pol II抑制激活细胞死亡独立于转录损失
RNA Pol ii介导的转录是真核生物生命所必需的。虽然转录缺失被认为是普遍致命的,但促进细胞死亡的相关机制尚不清楚。在这里,我们表明RNA Pol II活性丧失后的死亡不是由基因表达失调引起的。相反,它发生在Rbp1的低磷酸化形式(也称为RNA Pol IIA)丢失的反应中。RNA Pol IIA的缺失只会激活细胞凋亡,而Rpb1转录失活版本的表达可以挽救细胞活力。利用功能基因组学,我们确定了RNA Pol IIA缺失后导致死亡的机制,我们称之为Pol II降解依赖性凋亡反应(PDAR)。利用PDAR的遗传依赖性,我们确定了临床使用的药物,这些药物的致死率归因于PDAR依赖机制。我们的发现揭示了一种凋亡信号反应,它有助于广泛的抗癌治疗的疗效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


