ER-phagy and proteostasis defects prime pancreatic epithelial state changes in KRAS-mediated oncogenesis
IF 8.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
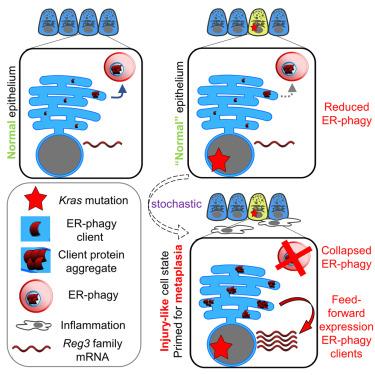
Pre-malignant transformation of pancreatic acinar cells by oncogenic Kras is dependent upon stochastic emergence of metaplastic cell states. Here, we reveal that an early, transcriptionally mediated effect of Kras is sporadic failure of proteostatic endoplasmic reticulum (ER)-phagy. Genetically altered mice deficient in ER-phagy demonstrate that this event cooperates with Kras to drive acinar-ductal metaplasia (ADM) and subsequent cancer. Mechanistically, proteomics and high-resolution imaging uncover pathologic aggregation of a subset of ER proteins, including the injury marker REG3B, resulting from failure to physically interact with the ER-phagy receptor CCPG1. Spatial transcriptomics demonstrate that the appearance of sporadic intracellular aggregates upon Kras activation marks rare acinar cells existing in an injured, ADM-primed state. Importantly, engineered mutants of REG3B establish that aggregate formation is sufficient to directly engender this epithelial cell state. Pancreatic cancer can thus arise from stochastic pathologic protein aggregates that are influenced by, and cooperate with, an oncogene.
在kras介导的肿瘤发生中,er吞噬和蛋白酶抑制缺陷导致胰腺上皮状态的改变
癌性Kras对胰腺腺泡细胞的癌前转化依赖于化生细胞状态的随机出现。在这里,我们揭示了Kras的早期转录介导作用是蛋白酶抑制内质网(ER)吞噬的散发性失败。缺乏er吞噬的转基因小鼠表明,这一事件与Kras协同驱动腺泡导管化生(ADM)和随后的癌症。在机制上,蛋白质组学和高分辨率成像揭示了ER蛋白亚群的病理聚集,包括损伤标志物REG3B,这是由于无法与ER吞噬受体CCPG1物理相互作用而导致的。空间转录组学表明,Kras激活时零星细胞内聚集体的出现标志着罕见的腺泡细胞存在于损伤的adm启动状态。重要的是,REG3B的工程突变体证实了聚集体的形成足以直接产生这种上皮细胞状态。因此,胰腺癌可由受癌基因影响或与癌基因合作的随机病理性蛋白聚集体引起。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


