A novel mycotoxin-degrading enzyme complex can biodegrade AFB1, DON, and ZEN co-contamination in both in vitro and in vivo experiments
IF 2.4
4区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
A novel mycotoxin-degrading enzyme complex (MDE), developed via a microcapsule coating process and containing three degrading enzymes, exhibits the ability to biodegrade aflatoxin B1 (AFB1), deoxynivalenol (DON) and zearalenone (ZEN). This study aims to evaluate the efficacy of MDE against AFB1, DON, and ZEN through in vitro and in vivo experiments. In vitro simulated digestion experiments revealed that the MDE degraded the concentrations of AFB1, DON, and ZEN by 76.27 %, 74.04 %, and 60.77 %, respectively. In vivo experiments were conducted using 39 one-day-old male Cobb broilers allocated into three groups: a basal diet group (BD; CON), a BD group supplemented with 50 μg/kg AFB1, 3.0 mg/kg DON, and 1.5 mg/kg ZEN (Toxins), and a BD plus Toxins diet with 0.02 % MDE (Toxins + MDE), with the experiment lasting for 14 days. Compared to the Toxins group, the Toxins + MDE group showed a tendency to increase (P = 0.09) the body weight on day 7. Moreover, the Toxins treatment increased the serum alanine aminotransferase (ALT) activities, aspartate aminotransferase (AST) activities, and blood urea nitrogen (BUN) concentrations, and decreased creatinine (CREA) concentrations. Interestingly, dietary supplementation with 0.02 % MDE alleviated these adverse effects. Additionally, the Toxins and Toxins + MDE groups exhibited slight lymphocytic infiltration and mucosal epithelial detachment in the glandular stomach and the villi layer. Notably, dietary supplementation with 0.02 % MDE decreased gastric AFB1, DON, and ZEN concentrations by 40.1 %, 37.7 %, and 29.1 %, respectively. In conclusion, MDE effectively degrades the concentrations of AFB1, DON, and ZEN through in vitro simulated pig digestion and in vivo chick experiments.
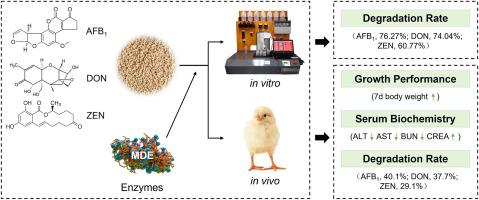
在体外和体内实验中,一种新的真菌毒素降解酶复合物可以生物降解AFB1, DON和ZEN共污染。
通过微胶囊包衣工艺制备了一种含有三种降解酶的新型真菌毒素降解酶复合物(MDE),该复合物具有生物降解黄曲霉毒素B1 (AFB1)、脱氧雪腐镰刀菌醇(DON)和玉米赤霉烯酮(ZEN)的能力。本研究旨在通过体外和体内实验,评价MDE对AFB1、DON和ZEN的抑制作用。体外模拟消化实验表明,MDE对AFB1、DON和ZEN的降解率分别为76.27%、74.04%和60.77%。体内试验选用39只1日龄雄性科布肉鸡,分为3组:基础饲粮组(BD);对照组为添加50 μg/kg AFB1、3.0 mg/kg DON和1.5 mg/kg ZEN(毒素)的BD组和添加0.02% MDE(毒素+MDE)的BD +毒素饲粮,试验期14 d。与Toxins组相比,毒素+MDE组在第7天有增加体重的趋势(P = 0.09)。毒素处理提高了血清丙氨酸转氨酶(ALT)、天冬氨酸转氨酶(AST)活性和尿素氮(BUN)浓度,降低了肌酐(CREA)浓度。有趣的是,添加0.02% MDE的膳食补充剂减轻了这些不良反应。此外,毒素和毒素+MDE组在腺胃和绒毛层有轻微的淋巴细胞浸润和粘膜上皮脱离。值得注意的是,饲粮中添加0.02% MDE可使胃中AFB1、DON和ZEN浓度分别降低40.1%、37.7%和29.1%。综上所述,通过体外模拟猪消化和鸡体内实验,MDE能有效降解AFB1、DON和ZEN的浓度。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Toxicon
医学-毒理学
CiteScore
4.80
自引率
10.70%
发文量
358
审稿时长
68 days
期刊介绍:
Toxicon has an open access mirror Toxicon: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review. An introductory offer Toxicon: X - full waiver of the Open Access fee.
Toxicon''s "aims and scope" are to publish:
-articles containing the results of original research on problems related to toxins derived from animals, plants and microorganisms
-papers on novel findings related to the chemical, pharmacological, toxicological, and immunological properties of natural toxins
-molecular biological studies of toxins and other genes from poisonous and venomous organisms that advance understanding of the role or function of toxins
-clinical observations on poisoning and envenoming where a new therapeutic principle has been proposed or a decidedly superior clinical result has been obtained.
-material on the use of toxins as tools in studying biological processes and material on subjects related to venom and antivenom problems.
-articles on the translational application of toxins, for example as drugs and insecticides
-epidemiological studies on envenoming or poisoning, so long as they highlight a previously unrecognised medical problem or provide insight into the prevention or medical treatment of envenoming or poisoning. Retrospective surveys of hospital records, especially those lacking species identification, will not be considered for publication. Properly designed prospective community-based surveys are strongly encouraged.
-articles describing well-known activities of venoms, such as antibacterial, anticancer, and analgesic activities of arachnid venoms, without any attempt to define the mechanism of action or purify the active component, will not be considered for publication in Toxicon.
-review articles on problems related to toxinology.
To encourage the exchange of ideas, sections of the journal may be devoted to Short Communications, Letters to the Editor and activities of the affiliated societies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


