Lack of validated blood pressure devices for use in pregnancy available from Australian pharmacies
IF 4.6
2区 医学
Q1 PERIPHERAL VASCULAR DISEASE
引用次数: 0
Abstract
Blood pressure monitoring is a critical aspect of prenatal care, as hypertension during pregnancy can lead to serious complications such as preeclampsia, eclampsia, and other hypertensive disorders. Automatic blood pressure devices are widely used for home monitoring due to their convenience and ease of use. The use of validated automated blood pressure monitors is recommended by the International Society of Hypertension for home blood pressure measurements, and automatic devices require accuracy validation among people who are pregnant before they are recommended for use in pregnancy. This study evaluated availability of such devices from 18 Australian pharmacies. Only four devices (4/54, 7%) were validated for pregnancy and were more expensive than devices validated for the general population (14/54, 26%) and non-validated devices (40/54, 74%). Additionally, limited labelling and information was available to assist consumers to make informed purchasing decisions about home blood pressure devices for use in pregnancy. Increased availability, clear labelling and consumer education could help ensure use of appropriate blood pressure devices in pregnancy. Automatic blood pressure devices require additional accuracy validation for use in pregnancy. We found only four devices (4/54, 7%) were validated for pregnancy, which were more expensive than non-validated devices. Increased education could help ensure use of appropriate blood pressure devices in pregnancy.
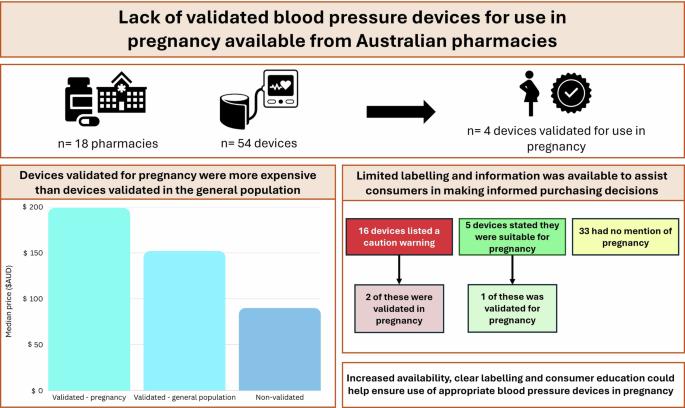
缺乏有效的血压设备用于妊娠可从澳大利亚药房。
血压监测是产前护理的一个重要方面,因为怀孕期间的高血压可导致严重的并发症,如先兆子痫、子痫和其他高血压疾病。自动血压仪由于使用方便,被广泛应用于家庭监测。国际高血压学会推荐使用经过验证的自动血压监测仪进行家庭血压测量,自动设备在推荐用于怀孕前需要在孕妇中进行准确性验证。这项研究评估了18家澳大利亚药店的这种设备的可用性。只有4种设备(4/ 54,7%)是为妊娠验证的,比为一般人群验证的设备(14/ 54,26%)和未验证的设备(40/ 54,74%)更昂贵。此外,有限的标签和信息,以帮助消费者作出明智的购买决定,家用血压装置用于怀孕。增加可获得性,清晰的标签和消费者教育可以帮助确保在怀孕期间使用适当的血压装置。自动血压装置需要额外的准确性验证用于妊娠。我们发现只有4种设备(4/ 54,7 %)被验证用于妊娠,这比未经验证的设备更昂贵。加强教育可以帮助确保在怀孕期间使用适当的血压设备。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Hypertension Research
医学-外周血管病
CiteScore
7.40
自引率
16.70%
发文量
249
审稿时长
3-8 weeks
期刊介绍:
Hypertension Research is the official publication of the Japanese Society of Hypertension. The journal publishes papers reporting original clinical and experimental research that contribute to the advancement of knowledge in the field of hypertension and related cardiovascular diseases. The journal publishes Review Articles, Articles, Correspondence and Comments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


