PBPK-PD model for predicting pharmacokinetics, tumor growth inhibition, and toxicity risks of topoisomerase inhibitor ADCs in mice and humans
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Background
Antibody-drug conjugates (ADCs) are composed of monoclonal antibodies conjugated to potent cytotoxic payloads via linkers for cancer treatment. However, the ADCs are also associated with the risk of drug-induced interstitial lung disease (ILD), primarily due to off-target payload distribution.
Objectives and Methods
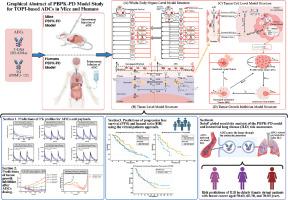
This study aimed to develop a whole-body physiologically based pharmacokinetic and pharmacodynamic (PBPK-PD) model to simultaneously predict the pharmacokinetics, therapeutic efficacy and associated risks of ILD using trastuzumab deruxtecan (T-DXd) and sacituzumab govitecan (SG) as model drugs. The model was initially validated in corresponding tumor-bearing mice and subsequently scaled up to breast cancer patients. Plasma concentrations of ADCs and their released payloads as well as progression-free survival (PFS) following treatment with the ADCs were simulated in patients using the developed PBPK-PD model.
Results
The results showed that most of the observed plasma concentrations of ADCs and their payloads fell within the 5th–95th percentiles derived from simulations of 1000 virtual patients, with the predicted AUC0-t and Cmax were all within 0.5-fold to 2-fold of the observations. The predicted PFS values were also consistent with clinical observations. Data from Sobol’ global sensitivity analysis showed that tumor surface antigen expression levels and the internalization rate of the antigen-ADC complex are the primary factors influencing ADCs distribution. A relationship between payload concentration in lungs and the risk of ILD was also documented, showing that the risk increased with advancing patient age and elevated dosage.
Conclusions
All these results give a conclusion that the developed PBPK-PD model may be applied to predict the pharmacokinetics and therapeutical efficacy of T-DXd and SG as well as risk of ILD.
PBPK-PD模型预测拓扑异构酶抑制剂adc在小鼠和人体内的药代动力学、肿瘤生长抑制和毒性风险
背景:抗体-药物偶联物(adc)由单克隆抗体通过连接物偶联到有效的细胞毒性有效载荷组成,用于癌症治疗。然而,adc也与药物性间质性肺疾病(ILD)的风险相关,主要是由于脱靶载荷分布。目的和方法:本研究以曲妥珠单抗德鲁德替康(T-DXd)和舒妥珠单抗govitecan (SG)为模型药物,建立基于生理的全身药代动力学和药效学(PBPK-PD)模型,同时预测ILD的药代动力学、疗效和相关风险。该模型最初在相应的荷瘤小鼠中得到验证,随后扩大到乳腺癌患者。使用开发的PBPK-PD模型模拟adc治疗后患者的血浆浓度及其释放的有效载荷以及无进展生存期(PFS)。结果:通过对1000例虚拟患者的模拟,结果显示,大部分adc的血浆浓度及其有效载荷均在5- 95个百分位数范围内,预测的AUC0-t和Cmax均在0.5- 2倍范围内。预测的PFS值也与临床观察一致。Sobol全球敏感性分析数据显示,肿瘤表面抗原表达水平和抗原- adc复合物内化率是影响adc分布的主要因素。肺部负荷浓度与ILD风险之间的关系也有文献记载,表明风险随着患者年龄的增长和剂量的增加而增加。结论:所建立的PBPK-PD模型可用于预测T-DXd和SG的药代动力学、疗效及ILD的发生风险。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


