The role of Fezolinetant in fear memory consolidation
IF 2.6
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
Abstract
Modulation of fear memories has important implications for the treatment of different psychiatric disorders including fear-based disorders such as posttraumatic stress disorder (PTSD). The Tachykinin2 (Tac2)/Neurokinin B (NkB)/neurokinin 3 receptor (Nk3R) pathway is a potential candidate for treating fear-based disorders built on animal and human studies. Here we demonstrate that the Nk3R antagonist Fezolinetant presents sex-divergent effects in the processing of fear memories in mice exposed to cued-fear conditioning. Fezolinetant (1 and 10 mg/kg, intraperitoneally (i.p.)) administered 30 min after the fear acquisition impairs the fear memory consolidation in male mice, as measured by fear expression 24 h later, showing no effects in naturally cycling female mice, with no monitoring the estrous cycle. However, when the estrous cycle was monitored, Fezolinetant (1 and 10 mg/kg, i.p.) enhanced fear memory consolidation during the proestrus phase, which suggests an effect dependent on high levels of the sex hormones estradiol and/or progesterone. Considering that Fezolinetant is currently a treatment for hot flashes in menopausal women, our results could be rapidly translated into clinical trials focused on treating or preventing fear-based disorders. Thus, these findings support the role of Tac2/NkB/Nk3R in fear memory consolidation and emphasize the importance of considering sex differences in the neurobiology of memory-related processes.
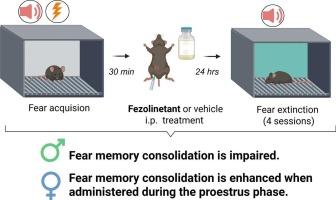
非唑啉肽在恐惧记忆巩固中的作用
恐惧记忆的调节对不同精神疾病的治疗具有重要意义,包括创伤后应激障碍(PTSD)等基于恐惧的疾病。Tachykinin2 (Tac2)/Neurokinin B (NkB)/ Neurokinin 3受体(Nk3R)通路是基于动物和人类研究建立的治疗基于恐惧的疾病的潜在候选途径。在这里,我们证明了Nk3R拮抗剂Fezolinetant在暴露于提示-恐惧条件反射的小鼠的恐惧记忆加工中呈现性别差异效应。在恐惧习得30分钟后给予Fezolinetant(1和10 mg/kg,腹腔注射)对雄性小鼠的恐惧记忆巩固有损害,24小时后通过恐惧表达测量,对自然循环的雌性小鼠没有影响,没有监测发情周期。然而,当监测发情周期时,非唑啉坦(1和10 mg/kg, i.p)增强了发情前期的恐惧记忆巩固,这表明其作用依赖于高水平的性激素雌二醇和/或黄体酮。考虑到Fezolinetant目前用于治疗更年期妇女的潮热,我们的结果可以迅速转化为临床试验,重点是治疗或预防基于恐惧的疾病。因此,这些发现支持了Tac2/NkB/Nk3R在恐惧记忆巩固中的作用,并强调了在记忆相关过程的神经生物学中考虑性别差异的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


