Apolipoprotein CIII in statin-treated type 2 diabetic patients: Its implications for plaque progression and instability: The pre-specified analysis from the OPTIMAL randomized controlled trial
IF 5.7
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Aims
Apolipoprotein CIII (ApoCIII) has a variety of proatherogenic properties. Given that hyperglycemia induces ApoCIII transcription, this apolipoprotein may promote coronary atherosclerosis in type 2 diabetic patients. We aimed to elucidate whether ApoCIII affects plaque progression and instability in statin-treated type 2 diabetic patients.
Methods and results
The OPTIMAL study was a prospective randomized controlled trial that employed serial NIRS/IVUS imaging to evaluate the efficacy of glycemic control on coronary atherosclerosis in 94 statin-treated type 2 diabetic patients (UMIN000036721). Of these, 78 patients with both ApoCIII levels and NIRS/IVUS images at baseline and week 48 were analyzed.
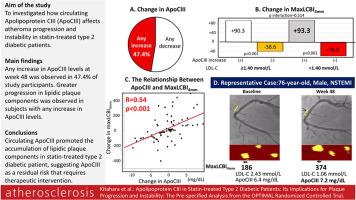
Any increase in ApoCIII levels at week 48 was observed in 47.4 % of study participants. On-treatment LDL-C levels did not differ among participants with and without any increase in ApoCIII levels (1.76 ± 0.55 vs. 1.74 ± 0.55 mmol/L, p = 0.91). Serial changes in IVUS-derived atheroma volume were similar between two groups (−0.7 ± 2.2 vs. −2.4 ± 1.6 mm3, p = 0.51). However, greater progression in NIRS-derived maxLCBI4mm was observed in those with any increase in ApoCIII levels (91.2 ± 24.8 vs. −44.2 ± 23.5, p < 0.001). Even after adjusting for clinical characteristics, maxLCBI4mm in participants with any increase in ApoCIII levels still progressed (87.0 ± 24.9 vs. −44.2 ± 23.5, p < 0.001). Moreover, maxLCBI4mm less likely regressed in patients with any increase in ApoCIII levels (16.2 vs. 80.5 %, p < 0.001). Even in those achieving on-treatment LDL-C<1.4 mmol/L, maxLCBI4mm progressed in association with any increase in ApoCIII levels.
Conclusion
Circulating ApoCIII promoted the accumulation of lipidic plaque components in statin-treated type 2 diabetic patient, suggesting ApoCIII as a residual risk that requires therapeutic intervention.
他汀类药物治疗的2型糖尿病患者的载脂蛋白CIII:其对斑块进展和不稳定性的影响:来自OPTIMAL随机对照试验的预先指定分析
载脂蛋白CIII (ApoCIII)具有多种促动脉粥样硬化特性。鉴于高血糖诱导ApoCIII转录,该载脂蛋白可能促进2型糖尿病患者冠状动脉粥样硬化。我们的目的是阐明ApoCIII是否影响他汀类药物治疗的2型糖尿病患者的斑块进展和不稳定性。方法和结果OPTIMAL研究是一项前瞻性随机对照试验,采用连续NIRS/IVUS成像来评估94例他汀类药物治疗的2型糖尿病患者(UMIN000036721)的血糖控制对冠状动脉粥样硬化的疗效。其中,78例患者在基线和第48周同时具有ApoCIII水平和NIRS/IVUS图像。在第48周,47.4%的研究参与者ApoCIII水平升高。ApoCIII水平升高和未升高的受试者在治疗期间LDL-C水平无差异(1.76±0.55 vs 1.74±0.55 mmol/L, p = 0.91)。ivus衍生的动脉粥样硬化体积的系列变化在两组之间相似(- 0.7±2.2 vs - 2.4±1.6 mm3, p = 0.51)。然而,在ApoCIII水平升高的患者中,nirs衍生的maxLCBI4mm的进展更大(91.2±24.8 vs - 44.2±23.5,p <;0.001)。即使在调整临床特征后,ApoCIII水平增加的受试者的maxLCBI4mm仍在进展(87.0±24.9 vs - 44.2±23.5,p <;0.001)。此外,ApoCIII水平升高的患者maxLCBI4mm不太可能消退(16.2% vs 80.5%, p <;0.001)。即使在治疗期间LDL-C<达到1.4 mmol/L的患者中,maxLCBI4mm也随着ApoCIII水平的增加而增加。结论循环ApoCIII促进了他汀类药物治疗的2型糖尿病患者脂质斑块成分的积累,提示ApoCIII是一种残留风险,需要进行治疗干预。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Atherosclerosis
医学-外周血管病
CiteScore
9.80
自引率
3.80%
发文量
1269
审稿时长
36 days
期刊介绍:
Atherosclerosis has an open access mirror journal Atherosclerosis: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Atherosclerosis brings together, from all sources, papers concerned with investigation on atherosclerosis, its risk factors and clinical manifestations. Atherosclerosis covers basic and translational, clinical and population research approaches to arterial and vascular biology and disease, as well as their risk factors including: disturbances of lipid and lipoprotein metabolism, diabetes and hypertension, thrombosis, and inflammation. The Editors are interested in original or review papers dealing with the pathogenesis, environmental, genetic and epigenetic basis, diagnosis or treatment of atherosclerosis and related diseases as well as their risk factors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


