The role of mTOR signaling pathway in systemic lupus erythematosus and systemic vasculitis
IF 8.3
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
The mechanistic target of rapamycin (mTOR) is a central regulator of cellular proliferation, metabolism, survival and growth. The mTOR pathway consists of two protein complexes, mTORC1 and mTORC2, which have different sensitivities to rapamycin and cellular functions. mTOR regulates autophagy as well as the function and/or differentiation of many immune cells, including T cells, dendritic cells, natural killer cells, macrophages, neutrophils, and B cells. mTOR signaling is implicated in the pathogenesis of cancer, diabetes and several autoimmune diseases. In this review, we summarize how mTOR pathway may contribute to the pathogenesis of systemic lupus erythematosus (SLE) and systemic vasculitis. In SLE, mTOR activation induces activation of CD4 + T cells, skews differentiation towards Th17 cells resulting in Th17/Treg imbalance, increases production of IL-4 in CD4 − CD8− double-negative (DN) T cells, reduces the number of circulating CD8+ memory T cells, promotes B-cell proliferation, increases production of plasmacytes and secretion of autoantibodies, as well as activation of myeloid dendritic cells. In large vessel vasculitis, mTOR overactivity promotes endothelial cell growth, T cell differentiation towards Th1 and Th17 polarization, impairment of Tregs and activation of smooth muscle cell-derived myofibroblasts that contribute to arterial stenosis and ischemia, whereas in anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis, reduced activity of the mTOR signaling pathway is observed in neutrophils isolated during the active phase of the disease. Targeting mTOR pathway with rapamycin, rapalogues, or other mTOR inhibitors could be efficacious in the treatment of these complex autoimmune diseases.
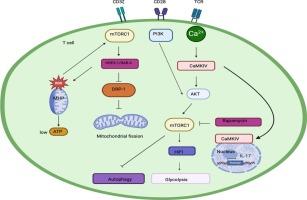
mTOR信号通路在系统性红斑狼疮和系统性血管炎中的作用
雷帕霉素(mTOR)的机制靶点是细胞增殖、代谢、生存和生长的中心调节剂。mTOR通路由mTORC1和mTORC2两种蛋白复合物组成,它们对雷帕霉素的敏感性和细胞功能不同。mTOR调节自噬以及许多免疫细胞的功能和/或分化,包括T细胞、树突状细胞、自然杀伤细胞、巨噬细胞、中性粒细胞和B细胞。mTOR信号与癌症、糖尿病和几种自身免疫性疾病的发病机制有关。本文就mTOR通路在系统性红斑狼疮(SLE)和系统性血管炎发病机制中的作用进行综述。在SLE中,mTOR激活诱导CD4 + T细胞激活,向Th17细胞分化导致Th17/Treg失衡,增加CD4 - CD8 -双阴性(DN) T细胞中IL-4的产生,减少循环CD8+记忆T细胞的数量,促进b细胞增殖,增加浆细胞的产生和自身抗体的分泌,以及骨髓树突状细胞的激活。在大血管炎中,mTOR过度活性促进内皮细胞生长、T细胞向Th1和Th17极化分化、Tregs损伤和平滑肌细胞源性肌成纤维细胞的激活,从而导致动脉狭窄和缺血,而在抗中性粒细胞胞浆自身抗体(ANCA)相关血管炎中,在疾病活动期分离的中性粒细胞中观察到mTOR信号通路活性降低。用雷帕霉素、雷帕衍生物或其他mTOR抑制剂靶向mTOR通路可能有效治疗这些复杂的自身免疫性疾病。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Autoimmunity reviews
医学-免疫学
CiteScore
24.70
自引率
4.40%
发文量
164
审稿时长
21 days
期刊介绍:
Autoimmunity Reviews is a publication that features up-to-date, structured reviews on various topics in the field of autoimmunity. These reviews are written by renowned experts and include demonstrative illustrations and tables. Each article will have a clear "take-home" message for readers.
The selection of articles is primarily done by the Editors-in-Chief, based on recommendations from the international Editorial Board. The topics covered in the articles span all areas of autoimmunology, aiming to bridge the gap between basic and clinical sciences.
In terms of content, the contributions in basic sciences delve into the pathophysiology and mechanisms of autoimmune disorders, as well as genomics and proteomics. On the other hand, clinical contributions focus on diseases related to autoimmunity, novel therapies, and clinical associations.
Autoimmunity Reviews is internationally recognized, and its articles are indexed and abstracted in prestigious databases such as PubMed/Medline, Science Citation Index Expanded, Biosciences Information Services, and Chemical Abstracts.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


