A patient-derived decellularized extracellular matrix hydrogel as a biomimetic scaffold for advanced 3D colorectal cancer modeling
IF 6
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2025-08-05
DOI:10.1016/j.bioadv.2025.214438
引用次数: 0
Abstract
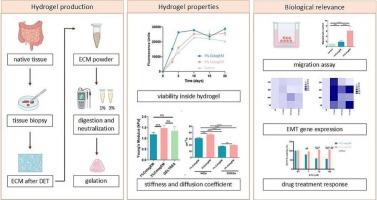
Colorectal cancer (CRC) is among the most prevalent cancers globally and is associated with a high mortality rate, particularly in advanced stages. In the realm of drug discovery, the use of innovative and highly translational pre-clinical CRC models is essential. Currently, the most relevant in vitro tumor approaches are three dimensional (3D) models. However, most 3D models of solid tumors are based either on synthetic materials or animal-derived commercial hydrogels, which fail to accurately mimic the biology of native tissues and originate from non-human sources. In contrast, hydrogels derived from human decellularized extracellular matrix (ECM) retain signaling cues from native tissue and represent a bioactive mechanical structure that can foster tumor cell growth in a tissue-specific 3D in vitro environment. Here, we demonstrated that patient-derived decellularized colon ECM can be processed into a hydrogel, producing the CologEM. CologEM formulation process preserved key ECM proteins, such as collagens, glycosaminoglycans and secreted bioactive molecules belonging to the family of cytokine, chemokine, interleukin, growth factors and ECM-remodeling enzyme. CologEM displayed a fibrous ultrastructure with interconnected pores, with notable differences observed between 1 % and 3 % (w/v) CologEM. Both 1 % and 3 % CologEM showed good biocompatibility, with 3 % CologEM demonstrating a higher propensity to induce a mesenchymal phenotype and resistance to antitumor drugs. In conclusion, CologEM is a suitable scaffold for 3D CRC models as it replicates critical characteristics of the tumor microenvironment. This model holds promise for facilitating the discovery and development of chemotropic drugs for cancer treatment.
一种患者来源的脱细胞细胞外基质水凝胶作为高级三维结肠直肠癌建模的仿生支架
结直肠癌(CRC)是全球最常见的癌症之一,死亡率高,特别是在晚期。在药物发现领域,使用创新和高度转化的临床前CRC模型是必不可少的。目前,最相关的体外肿瘤治疗方法是三维(3D)模型。然而,大多数实体肿瘤的3D模型要么是基于合成材料,要么是基于动物来源的商业水凝胶,这不能准确地模拟天然组织的生物学特性,而且来源于非人类来源。相比之下,来自人脱细胞细胞外基质(ECM)的水凝胶保留了来自天然组织的信号信号,并代表了一种生物活性机械结构,可以在组织特异性3D体外环境中促进肿瘤细胞生长。在这里,我们证明了患者来源的去细胞化结肠ECM可以加工成水凝胶,产生CologEM。CologEM的配方过程保留了关键的ECM蛋白,如胶原、糖胺聚糖等,并分泌了属于细胞因子、趋化因子、白细胞介素、生长因子和ECM重塑酶家族的生物活性分子。在1%和3% (w/v)的含量下,CologEM表现为纤维状超微结构,孔隙相互连接,差异显著。1%和3%的CologEM均表现出良好的生物相容性,其中3%的CologEM表现出更高的诱导间充质表型和抗肿瘤药物耐药性的倾向。综上所述,CologEM是3D CRC模型的合适支架,因为它复制了肿瘤微环境的关键特征。这种模式有望促进癌症治疗药物的发现和开发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


