Electrolyte-driven interphase stabilization in high-voltage sodium-ion full cells
IF 22
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
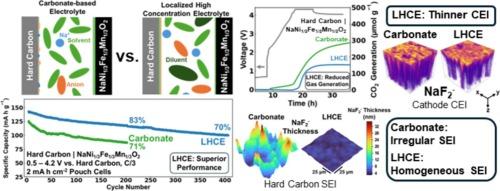
Sodium-ion batteries with a high-voltage O3-type layered oxide cathode paired with a hard carbon anode can offer high energy density; however, significant interfacial instabilities driven by electrode/electrolyte reactions limit a broader industrial adoption. Localized high concentration electrolytes (LHCEs) are a rational choice as they promote salt decomposition over solvent, forming stable, inorganic-rich electrode–electrolyte interphases (EEIs). We present here a comparison of high-voltage (4.2 V) hard carbon | NaNi1/3Fe1/3Mn1/3O2 pouch cells in LHCEs and in a standard carbonate-based electrolyte by (i) examining the influence of diluent choice on the electrochemical performance of LHCEs and (ii) investigating how the electrolyte chemistry affects the composition and structure of EEIs formed. Importantly, LHCEs demonstrate superior electrochemical performance, achieving 37 % higher capacity after 200 cycles (119 vs. 87 mA h g−1) compared to the carbonate-based electrolyte. The enhanced stabilization provided by LHCEs at the interface with high-voltage sodium layered oxide cathode is revealed by gas evolution measurements obtained through online electrochemical mass spectrometry (OEMS). Time-of-flight secondary ion mass spectrometry paired with focused ion beam and advanced statistical analyses reveal that the superior performance of LHCE stems from a robust, thin cathode electrolyte interphase formed on the sodium layered oxide cathode and a homogeneous solid electrolyte interphase formation on the hard carbon anode. This study highlights the critical importance of electrolyte design in interphase stabilization, which plays a key role in advancing sodium-ion batteries toward commercial viability.
高压钠离子电池中电解质驱动的间相稳定
采用高压o3型层状氧化物阴极和硬碳阳极的钠离子电池可以提供高能量密度;然而,由电极/电解质反应驱动的显著界面不稳定性限制了更广泛的工业应用。局部高浓度电解质(LHCEs)是一种合理的选择,因为它们促进盐的分解而不是溶剂,形成稳定的、富含无机的电极-电解质界面(EEIs)。在这里,我们比较了高压(4.2 V)硬碳| NaNi1/3Fe1/3Mn1/3O2袋状电池在LHCEs和标准碳酸基电解质中的性能,通过(i)研究稀释剂选择对LHCEs电化学性能的影响,以及(ii)研究电解质化学如何影响形成的eei的组成和结构。重要的是,lhce表现出优异的电化学性能,与碳酸盐基电解质相比,在200次循环后(119比87 mA h g−1)的容量提高了37%。通过在线电化学质谱(OEMS)获得的气体演化测量结果显示,LHCEs在高压氧化钠层状阴极界面上提供了增强的稳定性。飞行时间二次离子质谱法、聚焦离子束和先进的统计分析表明,LHCE的优越性能源于在钠层状氧化物阴极上形成的坚固、薄的阴极电解质界面和在硬碳阳极上形成的均匀的固体电解质界面。这项研究强调了电解质设计在相间稳定中的重要性,它在推动钠离子电池走向商业可行性方面起着关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today
工程技术-材料科学:综合
CiteScore
36.30
自引率
1.20%
发文量
237
审稿时长
23 days
期刊介绍:
Materials Today is the leading journal in the Materials Today family, focusing on the latest and most impactful work in the materials science community. With a reputation for excellence in news and reviews, the journal has now expanded its coverage to include original research and aims to be at the forefront of the field.
We welcome comprehensive articles, short communications, and review articles from established leaders in the rapidly evolving fields of materials science and related disciplines. We strive to provide authors with rigorous peer review, fast publication, and maximum exposure for their work. While we only accept the most significant manuscripts, our speedy evaluation process ensures that there are no unnecessary publication delays.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


