TCCA-Mediated Oxidative Deprotection of a Benzylamine
IF 3.5
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Benzyl-protected amines are mainly deprotected on scale using palladium-catalyzed hydrogenolysis. The present study evaluates a metal and hydrogen-free alternative based on the amine moiety oxidation to provide the corresponding imine via halogenation. It has been observed on a model substrate that inexpensive and nontoxic trichloroisocyanuric acid (TCCA) proved highly efficient as the oxidant before a simple base could readily deliver the desired imine to be hydrolyzed. A safety assessment of the process was further carried out and showed no specific alert regarding the chloramine intermediate formation.
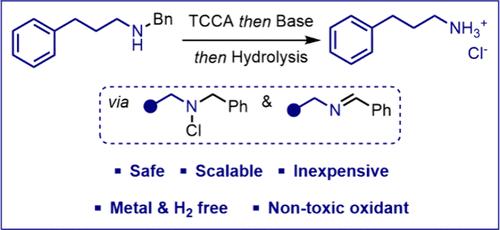
tcca介导的一种苯胺氧化脱保护作用
苯保护胺主要是用钯催化氢解法在水垢上脱保护的。本研究评估了一种基于胺部分氧化的金属和无氢替代品,通过卤化反应提供相应的亚胺。在模型底物上观察到,廉价无毒的三氯异氰尿酸(TCCA)被证明是高效的氧化剂,然后一个简单的碱可以很容易地传递所需的亚胺进行水解。对该工艺进行了进一步的安全评估,并没有显示有关氯胺中间体形成的具体警报。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


