Inhibition of tumor cell macropinocytosis driver DHODH reverses immunosuppression and overcomes anti-PD1 resistance
IF 26.3
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Tumor cells’ macropinocytosis sustains their growth under nutrient-limiting conditions. However, the metabolic regulation of cancer macropinocytosis in immune escape and its effect on immunotherapy remain unclear. Through the metabolism compound library and genome-wide CRISPR-Cas9 screenings, we identified dihydroorotate dehydrogenase (DHODH) as an essential driver of tumor cell macropinocytosis. DHODH sustained O-GlcNAcylation of the macropinocytic mediator neuropilin-1 (NRP1) and its membrane localization, mediating tumor cell macropinocytosis. Moreover, the DHODH-NRP1 axis-driven macropinocytosis increased intracellular amounts of lysine and tryptophan, which promoted glutarylation of the transcription factor class II transactivator (CIITA) to repress cancer cell major histocompatibility complex class II (MHC class II) expression. Pharmacological inhibition or genetic deletion of tumor cell-expressed DHODH potently recruited more immune cell infiltration and activated antitumor immunity in vivo, overcoming anti-programmed cell death protein 1 (PD1) resistance. High expression of DHODH and NRP1 in human breast and lung cancer tissues predicted patients’ poor prognosis. Therefore, targeting DHODH to inhibit tumor cell macropinocytosis provides a potential approach to reverse immunosuppression for improving cancer immunotherapy.
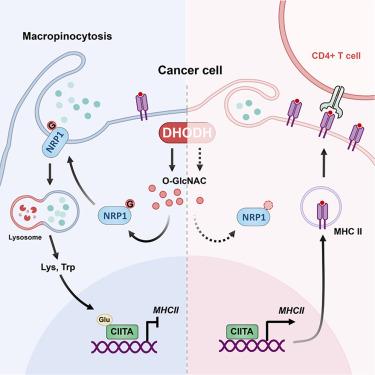
抑制肿瘤细胞巨噬细胞增多驱动因子DHODH逆转免疫抑制并克服抗pd1耐药性
肿瘤细胞的巨噬作用维持了它们在营养限制条件下的生长。然而,癌症巨噬细胞在免疫逃逸中的代谢调节及其对免疫治疗的影响尚不清楚。通过代谢化合物文库和全基因组CRISPR-Cas9筛选,我们确定了二氢酸脱氢酶(DHODH)是肿瘤细胞巨噬细胞症的重要驱动因素。DHODH维持巨噬细胞介质neuropilin-1 (NRP1)的o - glcn酰化及其膜定位,介导肿瘤细胞巨噬细胞作用。此外,DHODH-NRP1轴驱动的巨噬细胞增多症增加了细胞内赖氨酸和色氨酸的量,从而促进转录因子II类反激活子(CIITA)的戊二酰化,从而抑制癌细胞主要组织相容性复合体II类(MHC II类)的表达。药物抑制或基因缺失肿瘤细胞表达的DHODH可在体内招募更多免疫细胞浸润,激活抗肿瘤免疫,克服抗程序性细胞死亡蛋白1 (anti-programmed cell death protein 1, PD1)耐药性。人乳腺癌和肺癌组织中DHODH和NRP1的高表达预示着患者预后不良。因此,以DHODH为靶点抑制肿瘤细胞巨噬功能,为改善肿瘤免疫治疗提供了一种逆转免疫抑制的潜在途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


