Adsorption capacity of single and twin-tailed cationic and anionic surfactant-modified chitosan hydrogel beads for PAH removal from aqueous solutions
IF 6.3
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
This study presents a thorough investigation into the use of single and twin-tailed cationic and anionic surfactant-modified chitosan (SMCS) hydrogel beads as effective adsorbents for the elimination of hazardous polycyclic aromatic hydrocarbons (PAHs) from aqueous solutions. The Chitosan (CS) hydrogel beads were modified with single/twin-tailed anionic surfactants, sodium dodecyl sulfate (SDS) and sodium bis(2-ethylhexyl) sulfosuccinate (AOT), and cationic surfactants, dodecyltrimethylammonium bromide (DTAB) and didodecyldimethylammonium bromide (DDAB), to enhance their adsorption capacity of PAHs. The CS and SMCS beads were evaluated for their structural, mechanical, and adsorption properties using a range of techniques, including infrared spectroscopy (IR), energy-dispersive X-ray spectroscopy (EDX), rheometry, and field emission scanning electron microscopy (FESEM). Adsorption experiments of naphthalene (Nap), acenaphthene (Ace), and phenanthrene (Phe) on SMCS beads demonstrate that they have significantly higher adsorption capacities than CS beads, due to increase in hydrophobic interactions. Adsorption capacity followed the trend, Phen > Ace > Nap for all the beads revealing that twin-tailed SMCS bead possess much higher adsorption capacities (Qmax) compared to single-tailed SMCS beads. For twin tailed surfactants, the maximum adsorption capacities for Nap, Ace and Phe varied as CS-AOT (CS-DDAB): 430.0 (323.8) 611.60 (538.18) 633.39 (536.99) mg/g respectively, outperforming other reported hydrogel beads. The study highlights the simplicity, eco-friendliness, and enhanced performance of surfactant modification for developing high-efficiency adsorbents, paving the way for cost-effective solutions in water remediation.
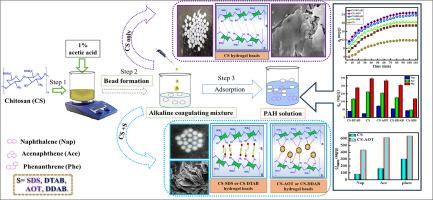
表面活性剂改性壳聚糖水凝胶珠对水溶液中多环芳烃的吸附性能
研究了单尾和双尾阳离子和阴离子表面活性剂修饰的壳聚糖(SMCS)水凝胶珠作为去除水溶液中有害多环芳烃(PAHs)的有效吸附剂。采用单尾/双尾阴离子表面活性剂十二烷基硫酸钠(SDS)和双(2-乙基己基)琥珀酸磺基钠(AOT)以及阳离子表面活性剂十二烷基三甲基溴化铵(DTAB)和二十二烷基二甲基溴化铵(DDAB)对壳聚糖(CS)水凝胶珠进行改性,以提高其对多环芳烃的吸附能力。利用红外光谱(IR)、能量色散x射线光谱(EDX)、流变学和场发射扫描电子显微镜(FESEM)等一系列技术,对CS和SMCS珠的结构、力学和吸附性能进行了评估。对萘(Nap)、苊(Ace)和菲(Phe)在SMCS珠上的吸附实验表明,由于疏水相互作用的增加,SMCS珠的吸附能力明显高于CS珠。吸附量遵循趋势,Phen >;Ace祝辞结果表明,与单尾SMCS珠相比,双尾SMCS珠具有更高的吸附能力(Qmax)。对于双尾表面活性剂,CS-AOT (CS-DDAB)对Nap、Ace和Phe的最大吸附量分别为430.0(323.8)、611.60(538.18)和633.39 (536.99)mg/g,优于其他已报道的水凝胶珠。该研究强调了表面活性剂改性的简单性、环保性和增强的性能,为开发高效吸附剂铺平了道路,为水修复提供了经济有效的解决方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Environmental Sciences-china
环境科学-环境科学
CiteScore
13.70
自引率
0.00%
发文量
6354
审稿时长
2.6 months
期刊介绍:
The Journal of Environmental Sciences is an international journal started in 1989. The journal is devoted to publish original, peer-reviewed research papers on main aspects of environmental sciences, such as environmental chemistry, environmental biology, ecology, geosciences and environmental physics. Appropriate subjects include basic and applied research on atmospheric, terrestrial and aquatic environments, pollution control and abatement technology, conservation of natural resources, environmental health and toxicology. Announcements of international environmental science meetings and other recent information are also included.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


