Cross-benzoin reaction between aliphatic aldehydes and aromatic acylals
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A chemoselective cross-benzoin reaction between aliphatic aldehydes and aromatic acylals as aldehyde equivalents is presented. A triazole-based N-heterocyclic carbene (NHC) catalyzed the reaction between straight-chain aliphatic aldehydes and (2-halophenyl)methylene diacetates (acylals) to afford α-acetoxy-α-(2-halophenyl) ketones in 14–84 % yields. For branched aliphatic aldehydes, the NHC derived from a trimethylthiazolium with less steric bulk in the vicinity of the carbene center functioned well along with the addition of 4-(dimethylamino)pyridine (DMAP) as a cocatalyst for the gradual generation of aromatic aldehydes from the corresponding acylals.
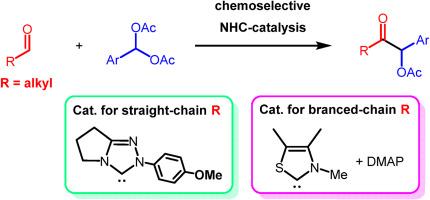
脂肪族醛和芳香酰基之间的交叉苯并苯甲酸反应
提出了一种脂肪族醛和芳香酰基醛作为醛等价物的化学选择性交叉苯甲酸反应。三唑基n -杂环羰基(NHC)催化直链脂肪醛与(2-卤代苯基)二乙酸亚甲酯(酰基)反应生成α-乙酰氧基-α-(2-卤代苯基)酮,产率为14 ~ 84%。对于支链脂肪族醛,由碳中心附近空间体积较小的三甲基噻唑衍生的NHC与4-(二甲氨基)吡啶(DMAP)的加入作为助催化剂,可以由相应的酰基逐渐生成芳香醛。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


