The interaction mechanism between piperine and myofibrillar protein was investigated using multi-spectral analysis, molecular docking, and molecular dynamics simulations
IF 7
2区 农林科学
Q1 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
Sichuan-style meat products are typical representatives of traditional and characteristic meat products in China. Piper longum L. is one of the commonly used spices in its processing, and piperine (PIP) is one of its main alkaloid components. The interaction between myofibrillar proteins (MPs) and PIP was investigated using multispectral analysis, molecular docking, and molecular dynamics simulations. Fluorescence quenching mechanisms reveal that the fluorescence of PIP on MPs is static, demonstrating a strong binding affinity. Circular dichroism analysis shows that interaction enhances the α-helical structure of MPs. The particle size of the formed complex initially increased before decreasing, while zeta potential first decreased, then increased. Additionally, molecular docking and dynamics simulations disclose that non-covalent interactions (electrostatic forces, van der Waals forces and hydrophobic interactions) are pivotal to the PIP-MPs interaction, with ARG202 and PHE622 being the most significant amino acid residues involved. We found that PIP's methyldioxophenyl selectively disrupts disulfide bonds in MHC, while its piperidine cyclic amide bond system promotes structural rearrangement in MPs through π-π stacking and hydrogen bonding networks. This research provides a theoretical foundation for optimizing natural spice applications in Sichuan-style meat products.
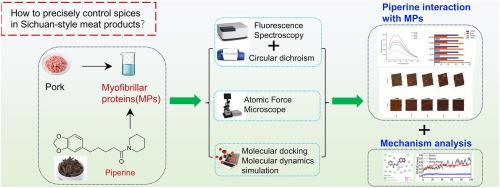
采用多光谱分析、分子对接、分子动力学模拟等方法研究胡椒碱与肌原纤维蛋白的相互作用机制
川式肉制品是中国传统肉制品和特色肉制品的典型代表。胡椒是加工中常用的香料之一,胡椒碱是其主要生物碱成分之一。通过多光谱分析、分子对接和分子动力学模拟研究了肌原纤维蛋白(MPs)和PIP之间的相互作用。荧光猝灭机制表明,PIP对MPs的荧光是静态的,显示出很强的结合亲和力。圆二色性分析表明,相互作用增强了MPs的α-螺旋结构。形成的配合物粒径先增大后减小,zeta电位先减小后增大。此外,分子对接和动力学模拟表明,非共价相互作用(静电力、范德华力和疏水相互作用)对PIP-MPs相互作用至关重要,其中ARG202和PHE622是最重要的氨基酸残基。我们发现PIP的甲基二氧苯选择性地破坏MHC中的二硫键,而其哌啶环酰胺键系统通过π-π堆叠和氢键网络促进MPs的结构重排。本研究为优化天然香料在川式肉制品中的应用提供了理论依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Current Research in Food Science
Agricultural and Biological Sciences-Food Science
CiteScore
7.40
自引率
3.20%
发文量
232
审稿时长
84 days
期刊介绍:
Current Research in Food Science is an international peer-reviewed journal dedicated to advancing the breadth of knowledge in the field of food science. It serves as a platform for publishing original research articles and short communications that encompass a wide array of topics, including food chemistry, physics, microbiology, nutrition, nutraceuticals, process and package engineering, materials science, food sustainability, and food security. By covering these diverse areas, the journal aims to provide a comprehensive source of the latest scientific findings and technological advancements that are shaping the future of the food industry. The journal's scope is designed to address the multidisciplinary nature of food science, reflecting its commitment to promoting innovation and ensuring the safety and quality of the food supply.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


