Lactobacillus extracellular vesicle-driven oxygen-releasing photothermal hydrogel reprograms macrophages and promotes angiogenesis to accelerate diabetic wound healing
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Chronic wound healing remains clinically challenging due to insufficient angiogenesis coupled with persistent inflammatory microenvironments. Macrophage M2 polarization plays a pivotal role in resolving inflammation and promoting angiogenesis. Capitalizing on scalability and translational advantages, extracellular vesicles derived from Lactobacillus bulgaricus (Lac-EVs) were employed to activate this mechanism. The anti-inflammatory and pro-angiogenic efficacy of Lac-EVs was initially confirmed through in vitro experiments. To support their delivery and function within the hostile diabetic wound microenvironment, a chitosan (CS)-based hydrogel incorporating haemoglobin (Hb)-polydopamine (PDA) complexes was engineered via Schiff base crosslinking with aldehyde-functionalised polyethylene glycol (CHO-PEG-CHO). This platform enabled stable delivery of Lac-EVs, supplemental oxygen release, and NIR-triggered photothermal functionality. In vitro studies demonstrated that the Lac-EVs-laden hydrogel (PCPH@Lac-EVs) effectively induced M2 macrophage polarization, enhanced endothelial cell migration, and promoted angiogenesis. In murine full-thickness diabetic wounds, PCPH@Lac-EVs combined with NIR irradiation achieved 99.3 % wound closure within 13 days, significantly outperforming untreated controls (72.3 %). Mechanistic analysis indicated that the accelerated healing resulted from synergistic enhancement of Lac-EV-mediated inflammation modulation and functional angiogenesis via oxygen release and mild photothermal stimulation. This study highlights the potential of Lac-EVs, delivered via a functional hydrogel, as a promising therapeutic strategy for diabetic wound treatment.
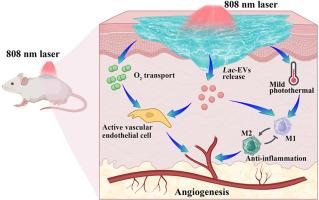
乳酸杆菌胞外囊泡驱动的释氧光热水凝胶重编程巨噬细胞,促进血管生成,加速糖尿病伤口愈合
由于血管生成不足加上持续的炎症微环境,慢性伤口愈合在临床上仍然具有挑战性。巨噬细胞M2极化在解决炎症和促进血管生成中起着关键作用。利用可扩展性和翻译优势,来自保加利亚乳杆菌的细胞外囊泡(lac - ev)被用来激活这一机制。通过体外实验初步证实了lac - ev的抗炎和促血管生成作用。为了支持它们在不利的糖尿病伤口微环境中的传递和功能,通过希夫碱与醛功能化聚乙二醇(CHO-PEG-CHO)交联,设计了一种含有血红蛋白(Hb)-聚多巴胺(PDA)复合物的壳聚糖(CS)基水凝胶。该平台实现了lac - ev的稳定输送、补充氧气释放和nir触发光热功能。体外研究表明,负载lac - ev的水凝胶(PCPH@Lac-EVs)可有效诱导M2巨噬细胞极化,增强内皮细胞迁移,促进血管生成。在小鼠糖尿病全层创面中,PCPH@Lac-EVs联合近红外照射在13天内创面愈合率达到99.3%,明显优于未治疗的对照组(72.3%)。机制分析表明,lac - ev介导的炎症调节和功能性血管生成通过氧气释放和轻度光热刺激协同增强,从而加速愈合。这项研究强调了lac - ev的潜力,通过功能性水凝胶输送,作为糖尿病伤口治疗的一种有前途的治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


