CRCs-CAFs crosstalk-targeted nano-delivery system reprograms tumor microenvironment for oxaliplatin resistance reversing and liver metastasis inhibition in colorectal cancer
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
The five-year survival rate of patients with colorectal cancer (CRC) liver metastasis is less than 30 %, and chemotherapy resistance and metastatic microenvironment remodeling are the current treatment bottlenecks. Cancer-associated fibroblasts (CAFs) in the tumor microenvironment (TME) form a “CRCs-CAFs crosstalk” with colorectal cancer cells (CRCs) by secreting dense extracellular matrix (ECM), free fatty acids (FFA), and pro-metastatic factors, driving a vicious cycle of drug resistance and metastasis. During liver metastasis, hepatic stellate cells (HSCs)-derived CAFs (HSC-CAFs) promote tumor metastasis by remodeling the pre-metastatic microenvironment. Based on clinical sample RNA sequencing and mouse single-cell sequencing to reveal ECM signal enrichment and CAFs activation characteristics, we innovatively constructed a nano-delivery system using hyaluronic acid-modified MIL-100 nanoparticles (OEMH NPs) co-loaded with oxaliplatin (OXA) and epigallocatechin gallate (EGCG). This system can target the CRCs-CAFs crosstalk through CD44 receptor: on the one hand, OEMH NPs can inhibit CAFs activation and reduce ECM deposition, improve drug penetration and down-regulate FFA metabolic reprogramming, reverse OXA resistance; on the other hand, OEMH NPs can block the transformation of HSCs to CAFs, down-regulate pro-metastatic factors such as VEGF/IL-11/ANG, induce vascular normalization, and reprogram the pre-metastatic microenvironment. This strategy can simultaneously achieve primary lesion drug sensitization and liver metastasis inhibition, providing a new paradigm for the treatment of advanced colorectal cancer to break through the traditional treatment dilemma through dual reprogramming of metabolism and microenvironment, and has significant clinical translation potential.
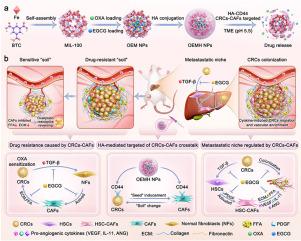
CRCs-CAFs串扰靶向纳米递送系统重编程肿瘤微环境逆转奥沙利铂耐药和抑制结直肠癌肝转移
结直肠癌(CRC)肝转移患者5年生存率不足30%,化疗耐药和转移性微环境重塑是目前治疗的瓶颈。肿瘤微环境(tumor microenvironment, TME)中的癌相关成纤维细胞(cancer -associated fibroblasts, CAFs)通过分泌致密的细胞外基质(extracellular matrix, ECM)、游离脂肪酸(free fatty acids, FFA)和促转移因子,与结直肠癌细胞(colorectal cancer cells, crc)形成“CRCs-CAFs串扰”,驱动耐药和转移的恶性循环。在肝转移过程中,肝星状细胞(hsc)衍生的CAFs (HSC-CAFs)通过重塑转移前微环境促进肿瘤转移。基于临床样本RNA测序和小鼠单细胞测序来揭示ECM信号富集和CAFs激活特性,我们创新地构建了透明质酸修饰的MIL-100纳米颗粒(OEMH NPs)共载奥沙利铂(OXA)和表没食子儿茶素没食子酸酯(EGCG)的纳米递送系统。该系统可通过CD44受体靶向CRCs-CAFs串扰:一方面,OEMH NPs可抑制CAFs活化,减少ECM沉积,提高药物渗透,下调FFA代谢重编程,逆转OXA耐药;另一方面,OEMH NPs可以阻断hsc向CAFs的转化,下调VEGF/IL-11/ANG等促转移因子,诱导血管正常化,并对转移前微环境进行重编程。该策略可同时实现原发病灶药物致敏和肝转移抑制,通过代谢和微环境双重重编程,为晚期结直肠癌治疗突破传统治疗困境提供了新范式,具有重要的临床转化潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


