Bioinspired porous core-shell microspheres with spatiotemporal delivery coordinate immunomodulatory-osteogenic coupling via NF-κB/P-STAT6 and Rho/MAPK signaling for enhanced calvarial regeneration
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Critical-sized calvarial defects remain a formidable clinical challenge due to dyssynchronous immunomodulation-osteogenesis coupling and unregulated growth factor release. Here, a bioinspired porous core-shell microsphere system (GCI@HPPS) is developed, integrating hydroxyapatite (HA)-loaded shell, surface-immobilized SDF-1α, and IGF-1-encapsulated cores to immunomodulate osteoimmune microenvironment and osteogenesis promotion. The hierarchical architecture achieved spatiotemporally programmed release: HA degradation-dependent mineralization, SDF-1α-mediated BMSC chemotaxis, and sustained IGF-1 delivery, mimicking natural bone repair cascades. Dual covalent/guest-host crosslinking (GelMA/Ac-β-CD) enhanced compressive strength, while polydopamine functionalization of microspheres conferred electroactivity, hydrophilicity, ROS/RNS scavenging (97.29 % ABTS•+ elimination), antibacterial efficacy (>99.8 %) and hemostasis. In vitro, GCI@HPPS mitigates oxidative stress, induces M2 macrophage polarization, and suppresses inflammatory cascades while concomitantly enhancing endogenous BMSC recruitment, proliferation, and osteogenic differentiation. Proteomics revealed a tetradic anti-inflammatory mechanisms of GCI@HPPS: NF-κB/P-JNK suppression, pro-inflammatory cytokines downregulation, mitochondrial oxidative modulation, and STAT6-driven M2 polarization. In vivo, GCI@HPPS achieved calvarial defect closure at 8 weeks through porous matrix-guided cellular infiltration, and SDF-1α/IGF-1-mediated chemotaxis, Rho/MAPK signaling pathway activation balancing osteoclast-osteoblast dynamics, stage-specific osteogenic induction and AGE-RAGE/VEGF-coupled angiogenesis-osteogenesis. This work pioneers a spatiotemporal delivery paradigm that coordinates inflammation modulation, stem cell recruitment, osteogenic differentiation, and mineralization phases, offering a promising approach for complex cranial reconstruction.
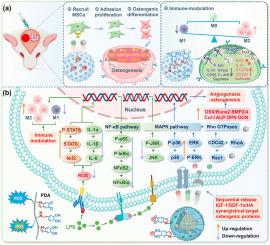
生物启发多孔核壳微球通过NF-κB/P-STAT6和Rho/MAPK信号协调免疫调节-成骨耦合,增强颅骨再生
由于不同步的免疫调节-成骨耦合和不调节的生长因子释放,临界尺寸的颅骨缺损仍然是一个巨大的临床挑战。本研究开发了一种生物启发的多孔核-壳微球系统(GCI@HPPS),将羟基磷灰石(HA)负载的壳、表面固定的SDF-1α和igf -1包封的核整合在一起,以免疫调节骨免疫微环境和促进成骨。分层结构实现了时空程序化释放:HA降解依赖的矿化,sdf -1α介导的BMSC趋化,以及持续的IGF-1递送,模拟自然骨修复级联。双共价/主-主交联(GelMA/Ac-β-CD)增强了抗压强度,而聚多巴胺功能化微球具有电活性、亲水性、ROS/RNS清除(97.29% ABTS•+消除)、抗菌功效(99.8%)和止血作用。在体外,GCI@HPPS减轻氧化应激,诱导M2巨噬细胞极化,抑制炎症级联反应,同时增强内源性BMSC募集、增殖和成骨分化。蛋白质组学揭示了GCI@HPPS四重抗炎机制:NF-κB/P-JNK抑制、促炎细胞因子下调、线粒体氧化调节和stat6驱动的M2极化。在体内,GCI@HPPS通过多孔基质引导的细胞浸润,以及SDF-1α/ igf -1介导的趋化性、Rho/MAPK信号通路激活平衡破骨细胞-成骨细胞动力学、阶段特异性成骨诱导和AGE-RAGE/ vegf偶联的血管生成-成骨,在8周时实现了颅骨缺损闭合。这项工作开创了一种时空传递模式,协调炎症调节、干细胞募集、成骨分化和矿化阶段,为复杂的颅骨重建提供了一种有前途的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


