Investigating conserved aromatic residues in ent-copalyl pyrophosphate synthases required for gibberellin phytohormone biosynthesis
IF 3.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Terpene synthases and cyclases catalyze carbocation cascade reactions, which have been hypothesized to be directed, in part, by aromatic residues via stabilization of specific intermediates through cation-π interactions towards specific product outcomes. Included in this are class II diterpene cyclases (DTCs), which are particularly widespread due to their role in initiating biosynthesis of gibberellin (GA) phytohormones but also function in production of a vast range of more specialized (labdane-related) diterpenoids. Indeed, the ent-copalyl pyrophosphate synthases (CPSs) required for GA biosynthesis are then conserved in all plants, with certain plant-associated bacteria that also produce GA containing potentially distantly related such CPSs as well. Building on the structure determined for the CPS from Arabidopsis thaliana (AtCPS), sequence comparison reveals that all seven aromatic residues in the active sites are conserved, suggesting these may play important roles in the catalyzed reaction. The role of these aromatic residues in directing product outcome was then examined via a series of substitutions for each in two representative examples, one from plants (AtCPS) and the other bacteria (EtCPS from Erwinia tracheiphila). Strikingly, substitution with even aliphatic residues had relatively little effect on product outcome, indicating more general structural roles for these aromatic groups. Accordingly, the role of aromatic residues in directing the carbocation cascade reactions catalyzed by at least such CPSs, if not also terpene cyclases and perhaps even synthases, requires additional evidence beyond simple presence in the active site, even when conserved.
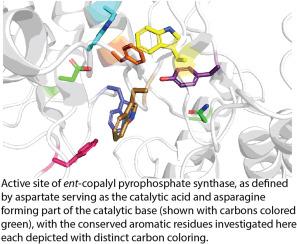
研究赤霉素植物激素生物合成所需的前-共alyl焦磷酸合成酶中保守的芳香残基
萜烯合成酶和环化酶催化碳正离子级联反应,该反应被假设在一定程度上是由芳香残基通过阳离子-π相互作用对特定产物产物的特定中间体的稳定所指导的。其中包括II类二萜环化酶(dtc),由于它们在启动赤霉素(GA)植物激素的生物合成中起作用而特别广泛,但也在生产广泛的更专门的(与labdane相关的)二萜中起作用。事实上,GA生物合成所需的前-共alyl焦磷酸合成酶(cps)在所有植物中都是保守的,某些与植物相关的细菌也会产生GA,这些细菌也可能含有与GA有远亲关系的cps。基于拟南芥(Arabidopsis thaliana, AtCPS) CPS的结构,序列比较发现活性位点的7个芳香残基都是保守的,这表明它们可能在催化反应中起重要作用。这些芳香残基在指导产物产出中的作用,然后通过在两个代表性的例子中进行一系列的替换,一个来自植物(AtCPS),另一个来自细菌(来自Erwinia tracheiphila)的EtCPS。引人注目的是,与脂肪族残基的取代对产物结果的影响相对较小,这表明这些芳香基团具有更普遍的结构作用。因此,芳香残基在至少由这些cps催化的碳阳离子级联反应中的作用,如果不是萜烯环化酶甚至合酶,则需要额外的证据,而不仅仅是在活性位点存在,即使是在保守的情况下。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


