Manganese transport systems of two enteric bacteria enhance their resistance to stress and enable the bacteria to evade the innate immune responses
IF 3.7
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Manganese (Mn) is an important element in bacteria-host interactions, exerting significant functions on both bacterial physiology and host immune responses. The importance of bacterial Mn transport systems in mediating bacterial stress resistance has been recognized, however, its role in modulating host innate immunity during infections remained elusive. This study aimed to explore the functions of the Mn transport proteins MntH and SitABCD in two enteric pathogens, Enterohemorrhagic Escherichia coli (EHEC) and Salmonella enterica serovar Typhimurium. We demonstrate that mutants deficient in these Mn uptake transporters (EHEC ΔmntH and S. typhimurium ΔmntHΔsitA) exhibit markedly reduced resistance to extreme environmental conditions like oxidative stress and impaired competitive advantages. Importantly, EHEC ΔmntH and S. typhimurium ΔmntHΔsitA elicited a significantly stronger innate immune response in macrophages compared to wild-type strains, indicating that MntH and SitABCD play a crucial role in inhibiting host immune activation. Specifically, we observed that Mn2+ enhanced the innate immune response to infection, and such an effect was abrogated in cGas−/− and Sting−/− macrophages. Importantly, MntH and SitABCD suppress innate immune response via the STING pathway. In conclusion, this study showed that the Mn transport systems in EHEC and S. typhimurium play important roles in modulating host immune responses, highlighting the importance of Mn availability in shaping the outcomes of enteropathogenic bacterial infections.
Importance
The manganese (Mn) transport systems MntH and SitABCD are crucial for bacterial survival. This study elucidates the role of Mn transport in enhancing bacterial resilience to oxidative stress and modulating the host's innate immune system, focusing on Enterohemorrhagic Escherichia coli (EHEC) and Salmonella enterica serovar Typhimurium. Our findings demonstrate that Mn uptake transporters not only confer stress resistance but also play a significant role in attenuating host immune activation through the STING signaling pathway. Mutants lacking MntH and SitABCD showed increased immune activation, suggesting these transporters help bacteria evade detection. The findings reveal that manganese not only enhances bacterial stress resistance but also modulates immune activation, thereby influencing infection outcomes.
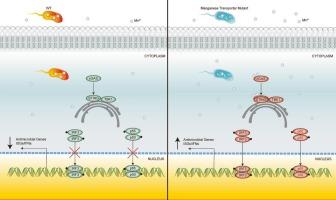
两种肠道细菌的锰转运系统增强了它们对应激的抵抗力,使细菌能够逃避先天免疫反应
锰(Mn)是细菌与宿主相互作用的重要元素,在细菌生理和宿主免疫反应中发挥重要作用。细菌Mn转运系统在介导细菌抗逆性中的重要性已被认识到,然而,其在感染期间调节宿主先天免疫的作用仍然难以捉摸。本研究旨在探讨锰转运蛋白MntH和SitABCD在肠出血性大肠杆菌(EHEC)和肠沙门氏菌血清型鼠伤寒杆菌两种肠道病原体中的功能。我们证明,缺乏这些锰摄取转运体的突变体(肠出血性大肠杆菌ΔmntH和鼠伤寒沙门氏菌ΔmntHΔsitA)对氧化应激等极端环境条件的抵抗力明显降低,竞争优势受损。重要的是,与野生型菌株相比,肠出血性大肠杆菌ΔmntH和鼠伤寒沙门氏菌ΔmntHΔsitA在巨噬细胞中引发了更强的先天免疫反应,这表明MntH和SitABCD在抑制宿主免疫激活中起着至关重要的作用。具体来说,我们观察到Mn2+增强了对感染的先天免疫反应,这种作用在cGas−/−和Sting−/−巨噬细胞中被消除。重要的是,MntH和SitABCD通过STING途径抑制先天免疫反应。总之,本研究表明,肠出血性大肠杆菌和鼠伤寒沙门氏菌中的锰转运系统在调节宿主免疫反应中发挥重要作用,突出了锰可获得性在形成肠致病性细菌感染结果中的重要性。锰(Mn)转运系统MntH和SitABCD对细菌存活至关重要。本研究阐明了锰转运在增强细菌抗氧化应激能力和调节宿主先天免疫系统中的作用,重点研究了肠出血性大肠杆菌(EHEC)和肠沙门氏菌血清型鼠伤寒沙门氏菌。我们的研究结果表明,Mn摄取转运体不仅赋予抗逆性,而且在通过STING信号通路减弱宿主免疫激活方面发挥重要作用。缺乏MntH和SitABCD的突变体显示免疫激活增加,表明这些转运蛋白帮助细菌逃避检测。研究结果表明,锰不仅可以增强细菌的抗逆性,还可以调节免疫激活,从而影响感染结果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cytokine
医学-免疫学
CiteScore
7.60
自引率
2.60%
发文量
262
审稿时长
48 days
期刊介绍:
The journal Cytokine has an open access mirror journal Cytokine: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
* Devoted exclusively to the study of the molecular biology, genetics, biochemistry, immunology, genome-wide association studies, pathobiology, diagnostic and clinical applications of all known interleukins, hematopoietic factors, growth factors, cytotoxins, interferons, new cytokines, and chemokines, Cytokine provides comprehensive coverage of cytokines and their mechanisms of actions, 12 times a year by publishing original high quality refereed scientific papers from prominent investigators in both the academic and industrial sectors.
We will publish 3 major types of manuscripts:
1) Original manuscripts describing research results.
2) Basic and clinical reviews describing cytokine actions and regulation.
3) Short commentaries/perspectives on recently published aspects of cytokines, pathogenesis and clinical results.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


