A self-powered photovoltaic colorimetric detector for sensing metal ions at ultralow concentrations
IF 10.61
Q3 Biochemistry, Genetics and Molecular Biology
引用次数: 0
Abstract
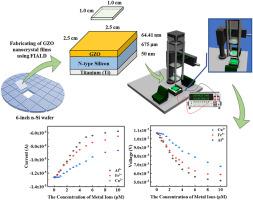
The accumulation of toxic metal ions from industrial activities poses significant environmental and health risks, thus necessitating the development of portable, rapid, and highly sensitive detection systems. We report a self-powered photovoltaic colorimetric sensor that is capable of detecting Al3+, Fe3+, and Cu2+ ions at nanomolar concentrations. Traditional spectrometer-based platforms are bulky and unsuitable for onsite applications, whereas conventional colorimetric sensors often suffer from limited sensitivity and poor reproducibility. To address these limitations, we utilize a rhodamine derivative (R6GH) that undergoes a ring-opening reaction upon interaction with target metal ions, which results in a visible color change under green LED illumination. The portable sensor integrates a Schottky junction that is fabricated by depositing gallium-doped zinc oxide (GZO) onto an n-type silicon substrate via atomic layer deposition, which enables the efficient conversion of optical signals into electrical outputs. The device operates in dual detection mode. In voltage mode, the detection limits are 16 nM for Al3+, 22 nM for Fe3+, and 41 nM for Cu2+. In current mode, the respective detection limits are 26, 18, and 34 nM. Compared with conventional chemosensors, this system offers an improvement in sensitivity of up to two orders of magnitude. Additionally, the sensor demonstrates excellent signal reproducibility, with a relative standard deviation (RSD) of less than 1.14 % across 560 switching cycles. The combination of high sensitivity, rapid response (<30 s), and stable, self-powered operation makes this device a promising candidate for real-time metal ion monitoring for the future of bioelectronic devices in healthcare.
用于感应超低浓度金属离子的自供电光伏比色检测器
工业活动产生的有毒金属离子的积累构成重大的环境和健康风险,因此有必要开发便携式、快速和高灵敏度的检测系统。我们报道了一种自供电光伏比色传感器,能够检测纳米摩尔浓度的Al3+, Fe3+和Cu2+离子。传统的基于光谱仪的平台体积庞大,不适合现场应用,而传统的比色传感器往往存在灵敏度有限和再现性差的问题。为了解决这些限制,我们利用罗丹明衍生物(R6GH)在与目标金属离子相互作用时发生开环反应,从而在绿色LED照明下产生可见的颜色变化。该便携式传感器集成了一个肖特基结,该结是通过原子层沉积将掺镓氧化锌(GZO)沉积在n型硅衬底上制成的,从而实现了光信号到电输出的有效转换。该设备工作在双检测模式。电压模式下,Al3+的检测限为16 nM, Fe3+的检测限为22 nM, Cu2+的检测限为41 nM。在当前模式下,检测限分别为26、18和34 nM。与传统的化学传感器相比,该系统的灵敏度提高了两个数量级。此外,该传感器具有出色的信号再现性,在560个开关周期内相对标准偏差(RSD)小于1.14%。高灵敏度、快速响应(30秒)和稳定的自供电操作相结合,使该设备成为未来医疗保健中生物电子设备实时金属离子监测的有希望的候选者。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biosensors and Bioelectronics: X
Biochemistry, Genetics and Molecular Biology-Biophysics
CiteScore
4.60
自引率
0.00%
发文量
166
审稿时长
54 days
期刊介绍:
Biosensors and Bioelectronics: X, an open-access companion journal of Biosensors and Bioelectronics, boasts a 2020 Impact Factor of 10.61 (Journal Citation Reports, Clarivate Analytics 2021). Offering authors the opportunity to share their innovative work freely and globally, Biosensors and Bioelectronics: X aims to be a timely and permanent source of information. The journal publishes original research papers, review articles, communications, editorial highlights, perspectives, opinions, and commentaries at the intersection of technological advancements and high-impact applications. Manuscripts submitted to Biosensors and Bioelectronics: X are assessed based on originality and innovation in technology development or applications, aligning with the journal's goal to cater to a broad audience interested in this dynamic field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


