Unveiling the interaction of uranyl and arsenate: Insights into the formation mechanisms of uranyl arsenate minerals
IF 6.3
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
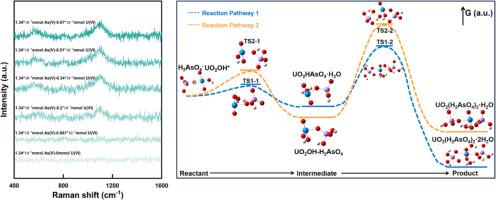
Uranyl arsenate minerals, which exhibit low solubility, serve as major sinks for U and As, playing a crucial role in controlling the mobility of U and As in the environment. However, the specific mechanisms underlying the formation of uranyl arsenate minerals have remained largely elusive. Herein, the formation pathway of the non-charged UO2(H2AsO4)2·nH2O0 complex was investigated to elucidate the early formation of the UO2(H2AsO4)2·nH2O mineral (where n represents the stoichiometric number of H2O), a representative uranyl arsenate mineral. Based on the combination experiments of U(VI) and As(V), our findings underscore the significant dependence of UO2(H2AsO4)2·nH2O0 formation on solution pH (4.0–10.0). Density functional theory (DFT) calculations reveal a two-step reaction involving two distinct pathways (Pathway 1 and Pathway 2) for the formation of UO2(H2AsO4)2·nH2O, and the intermediate was confirmed by in situ Raman and fluorescence spectroscopy. Specifically, the hydroxyl‑connected uranyl (UO2OH+) reacts with the protonated arsenate (H2AsO4-) species to form the intermediate UO2HAsO4·H2O (Pathway 1) or UO2OH![]() H2AsO4 (Pathway 2) with a U/As ratio of 1:1. Meanwhile, all the transition states also were obtained and the energy barrier suggested that the UO2(H2AsO4)2·2H2O0 formed by Pathway 1 is thermodynamically favored over Pathway 2, and may serve as the primary fundamental structural unit or precursor for the early formation of the UO2(H2AsO4)2·nH2O mineral phase. Altogether, this study contributes to advancing the understanding of the formation of uranyl arsenate minerals at the molecular scale and provides a theoretical basis for predicting and regulating uranium and arsenic mobilization in their coexisting environment.
H2AsO4 (Pathway 2) with a U/As ratio of 1:1. Meanwhile, all the transition states also were obtained and the energy barrier suggested that the UO2(H2AsO4)2·2H2O0 formed by Pathway 1 is thermodynamically favored over Pathway 2, and may serve as the primary fundamental structural unit or precursor for the early formation of the UO2(H2AsO4)2·nH2O mineral phase. Altogether, this study contributes to advancing the understanding of the formation of uranyl arsenate minerals at the molecular scale and provides a theoretical basis for predicting and regulating uranium and arsenic mobilization in their coexisting environment.
揭示铀酰和砷酸盐的相互作用:对铀酰砷酸盐矿物形成机制的见解
砷酸铀酰矿物具有较低的溶解度,是铀和砷的主要汇,对控制铀和砷在环境中的迁移起着至关重要的作用。然而,形成铀酰砷酸盐矿物的具体机制在很大程度上仍然难以捉摸。本文研究了不带电的UO2(H2AsO4)2·nH2O配合物的形成途径,阐明了UO2(H2AsO4)2·nH2O矿物(其中n代表H2O的化学计量数)的早期形成,UO2(H2AsO4)2·nH2O是一种代表性的铀酰砷酸盐矿物。基于U(VI)和As(V)的组合实验,我们的研究结果强调了UO2(H2AsO4)2·nh2o的形成对溶液pH(4.0-10.0)的显著依赖。密度泛函理论(DFT)计算表明,UO2(H2AsO4)2·nH2O的形成涉及两个不同的途径(途径1和途径2),并通过原位拉曼光谱和荧光光谱证实了中间体的存在。具体来说,羟基连接的铀酰(UO2OH+)与质子化的砷酸盐(H2AsO4-)反应形成中间产物UO2HAsO4·H2O(途径1)或UO2OHH2AsO4(途径2),U/As比为1:1。同时,所有过渡态也都得到了,能量势垒表明,途径1形成的UO2(H2AsO4)2·2h2o在热力学上优于途径2,可能是早期形成UO2(H2AsO4)2·nH2O矿物相的主要基本结构单元或前体。综上所述,本研究有助于在分子尺度上推进对铀酰砷酸盐矿物形成的认识,并为预测和调控共存环境下铀和砷的运移提供理论依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Environmental Sciences-china
环境科学-环境科学
CiteScore
13.70
自引率
0.00%
发文量
6354
审稿时长
2.6 months
期刊介绍:
The Journal of Environmental Sciences is an international journal started in 1989. The journal is devoted to publish original, peer-reviewed research papers on main aspects of environmental sciences, such as environmental chemistry, environmental biology, ecology, geosciences and environmental physics. Appropriate subjects include basic and applied research on atmospheric, terrestrial and aquatic environments, pollution control and abatement technology, conservation of natural resources, environmental health and toxicology. Announcements of international environmental science meetings and other recent information are also included.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


