TDP-43 mediated oxidative stress induced mitochondrial dysfunction in neurons and hyperalgesia in sciatic nerve injured mice
IF 2.6
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
Abstract
Neuropathic pain (NP) is a chronic pain with a highly complex pathogenesis, in which oxidative stress and mitochondrial dysfunction play significant roles in its progression, but its underlying mechanism is still unclear. TAR DNA-binding protein 43 (TDP-43) is one of the DNA-binding protein contributing to the homeostasis of mitochondria. This study is to explore the role of TDP-43 in mitochondrial dysfunction and pain formation in a mouse model. Therefore, in the mouse sciatic nerve chronic constriction injury (CCI) model and the H2O2-induced oxidative stress damage model in N2a cells, we examined the expression of TDP-43, and assessed whether inhibiting TDP-43 alleviated oxidative stress induced mitochondrial dysfunction. Additionally, we examined whether knockdown of TDP-43 could alleviate nociceptive behavior in CCI mice. Our results revealed a time-dependent upregulation of TDP-43 expression in the lumbar spinal dorsal horn neurons of CCI mice. In both in vivo and in vitro experiments, inhibiting TDP-43 attenuates oxidative stress-induced alterations in mitochondrial membrane potential (ΔΨm) and optic atrophy 1 (opa1) expression—a key regulator of mitochondrial fission. Furthermore, intrathecal injection of siRNA to knock down TDP-43 alleviated hyperalgesia and allodynia in CCI mice. These data indicate that TDP-43 in spinal neurons may contribute to NP by impairing mitochondrial function induced by oxidative stress, which may provide a new potential target for the treatment of NP.
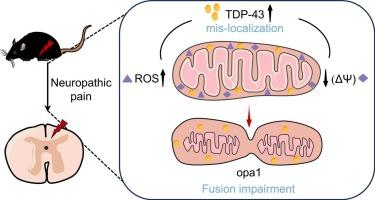
TDP-43介导的氧化应激诱导坐骨神经损伤小鼠神经元线粒体功能障碍和痛觉过敏
神经性疼痛(Neuropathic pain, NP)是一种发病机制高度复杂的慢性疼痛,氧化应激和线粒体功能障碍在其发病过程中起重要作用,但其发病机制尚不清楚。TAR dna结合蛋白43 (TDP-43)是参与线粒体内稳态的dna结合蛋白之一。本研究旨在探讨TDP-43在小鼠线粒体功能障碍和疼痛形成中的作用。因此,在小鼠坐骨神经慢性收缩损伤(CCI)模型和h2o2诱导的N2a细胞氧化应激损伤模型中,我们检测了TDP-43的表达,并评估抑制TDP-43是否减轻氧化应激诱导的线粒体功能障碍。此外,我们还检测了敲低TDP-43是否可以减轻CCI小鼠的伤害性行为。我们的研究结果揭示了CCI小鼠腰椎背角神经元中TDP-43表达的时间依赖性上调。在体内和体外实验中,抑制TDP-43可减弱氧化应激诱导的线粒体膜电位(ΔΨm)和视神经萎缩1 (opa1)表达的改变,后者是线粒体裂变的关键调节因子。此外,鞘内注射siRNA以降低TDP-43可减轻CCI小鼠的痛觉过敏和异位性疼痛。这些数据表明,脊髓神经元中的TDP-43可能通过损害氧化应激诱导的线粒体功能而参与NP,这可能为NP的治疗提供新的潜在靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


