Unveiling the surface hydroxyl-modulated effects for Cu1/γ-Al2O3 (110) in the direct oxidation of methane to methanol
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Surface microchemical environments of catalysts can bring potential modulations on catalytic processes, particularly for isolated active centers with high structural sensitivity. In this work, we focus on the direct methane (CH4)-to-methanol (CH3OH) (DMTM) oxidation with oxygen (O2), and theoretically decipher the critical effects of surface hydroxyl (–OH) species on single-atom copper supported on alumina (Cu1/γ-Al2O3). On the one hand, OH-(Cu, Al) (bonding with Cu and Al) can alleviate the orbital splitting of Cu1 3d and thus inhibit its migration. Simultaneously, the changes in orbital interactions also largely intensify O2 activation, thereby facilitating the formation of highly reactive Cu−O- species that can trigger CH4 homolysis with a lower energy barrier. On the other hand, OH-(Al, Al) (bonding with Al and Al) plays the role of a proton bridge and alters the generation channel of the second CH3OH molecule. Specifically, OH-(Al, Al) first releases H to combine with the foreign OH*, and the newly formed H2O* can weaken CH3* adsorption, subsequently mediating methanol formation and reconstructing OH-(Al, Al). In addition, the presence of –OH promotes the surface desorption of CH3OH and thus greatly prevents product overoxidation. This work provides insights into exploiting efficient DMTM catalysts from the perspective of surface functional group modifications.
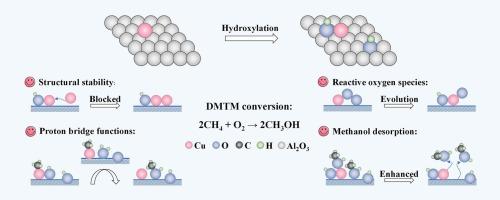
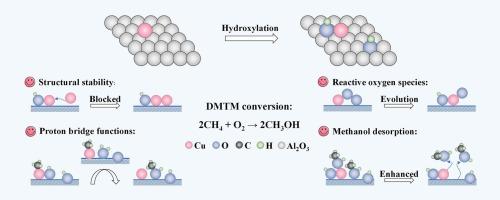
揭示了Cu1/γ-Al2O3(110)在甲烷直接氧化制甲醇过程中的表面羟基调节效应
催化剂的表面微化学环境可以对催化过程产生潜在的调节作用,特别是对具有高结构敏感性的孤立活性中心。在这项工作中,我们重点研究了甲烷(CH4)与氧(O2)直接氧化成甲醇(CH3OH) (DMTM),并从理论上解释了表面羟基(OH)物种对氧化铝负载单原子铜(Cu1/γ-Al2O3)的关键影响。一方面,OH (Cu, Al)(与Cu和Al成键)可以减轻Cu1 3d的轨道分裂,从而抑制其迁移;同时,轨道相互作用的变化也在很大程度上加强了O2的活化,从而促进了高活性Cu−O-物质的形成,从而以较低的能垒触发CH4均解。另一方面,OH (Al, Al)(与Al和Al成键)起到质子桥的作用,改变了第二个CH3OH分子的生成通道。具体来说,OH (Al, Al)首先释放H与外来OH*结合,新形成的H2O*可以减弱CH3*的吸附,随后介导甲醇的生成,重建OH (Al, Al)。此外,OH的存在促进了CH3OH的表面解吸,从而大大防止了产物的过氧化。这项工作为从表面官能团修饰的角度开发高效的DMTM催化剂提供了见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


