Genome-scale metabolic modelling in antimicrobial pharmacology: Present and future
IF 17.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
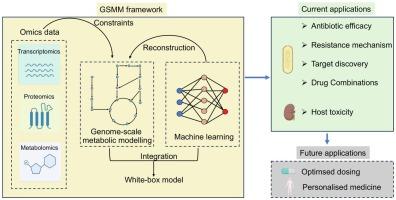
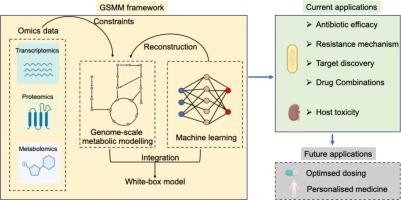
The persistent surge in antimicrobial resistance (AMR) has propelled the search for innovative strategies in antimicrobial use. Genome-scale metabolic modelling (GSMM) has emerged as a transformative tool in this quest, offering a comprehensive understanding of host and microbial metabolism and their interactions with antimicrobial agents. This review emphasises current advancements in the application of GSMM to antimicrobial pharmacology, highlighting its role in deciphering complex microbial and host responses to drug exposure, identifying novel therapeutic targets and optimising therapeutic options. We discuss how GSMM has elucidated mechanisms of drug action, resistance pathways, and off-target effects, providing a systems-level perspective that challenges the traditional “one drug, one target” approach. The integration of GSMM with high-throughput omics technologies and machine learning showcases its potential to refine predictions of drug efficacy, optimise dosing strategies, and minimise toxicity. We also address the challenges and future directions of GSMM, including its expansion to host-pathogen-drug interactions and personalised medicine. Ultimately, GSMM stands as a critical approach in modern antimicrobial research, with the potential to revolutionise the development of effective treatments against MDR pathogens.
基因组尺度代谢模型在抗菌药理学:现在和未来
抗菌素耐药性的持续激增推动了对抗菌素使用的创新战略的探索。基因组尺度代谢模型(GSMM)已经成为这一探索的变革工具,提供了对宿主和微生物代谢及其与抗菌药物相互作用的全面了解。这篇综述强调了GSMM在抗菌药理学应用方面的最新进展,强调了它在破译复杂的微生物和宿主对药物暴露的反应、确定新的治疗靶点和优化治疗方案方面的作用。我们讨论了GSMM如何阐明药物作用机制、耐药途径和脱靶效应,提供了挑战传统“一种药物,一种靶标”方法的系统级视角。GSMM与高通量组学技术和机器学习的整合展示了其改进药物疗效预测、优化给药策略和最小化毒性的潜力。我们还讨论了GSMM的挑战和未来方向,包括其扩展到宿主-病原体-药物相互作用和个性化医疗。最终,GSMM是现代抗微生物研究的基石,有可能彻底改变针对耐多药病原体的有效治疗方法的发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
28.10
自引率
5.00%
发文量
294
审稿时长
15.1 weeks
期刊介绍:
The aim of the Journal is to provide a forum for the critical analysis of advanced drug and gene delivery systems and their applications in human and veterinary medicine. The Journal has a broad scope, covering the key issues for effective drug and gene delivery, from administration to site-specific delivery.
In general, the Journal publishes review articles in a Theme Issue format. Each Theme Issue provides a comprehensive and critical examination of current and emerging research on the design and development of advanced drug and gene delivery systems and their application to experimental and clinical therapeutics. The goal is to illustrate the pivotal role of a multidisciplinary approach to modern drug delivery, encompassing the application of sound biological and physicochemical principles to the engineering of drug delivery systems to meet the therapeutic need at hand. Importantly the Editorial Team of ADDR asks that the authors effectively window the extensive volume of literature, pick the important contributions and explain their importance, produce a forward looking identification of the challenges facing the field and produce a Conclusions section with expert recommendations to address the issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


