Hypoimmune CD19 CAR T cells evade allorejection in patients with cancer and autoimmune disease
IF 20.4
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
Abstract
Off-the-shelf CAR T cells need to reliably escape allogeneic immune responses to become universal medicines. The primary T cell product SC291 was engineered with a CD19 CAR, T cell receptor alpha constant (TRAC) knockout, and the hypoimmune (HIP) edits of HLA depletion and CD47 overexpression. Here, we report exploratory immune analyses from the ARDENT (NCT05878184) and GLEAM (NCT06294236) trials with HIP-edited CD19 CAR T cells. Although there was an alloimmune response against HLA-replete subpopulations of SC291, we observed no de novo immune response against fully edited HIP CAR T cells in all patients, irrespective of the dose or the patient’s disease. The lack of antibodies against the HLA-replete CAR T cells was identified as a marker for deep tissue CD19 cell depletion, and all patients without such antibodies for 60 days showed concomitant B cell depletion in peripheral blood. The immune data presented support the reliability of the HIP concept to evade allorejection.
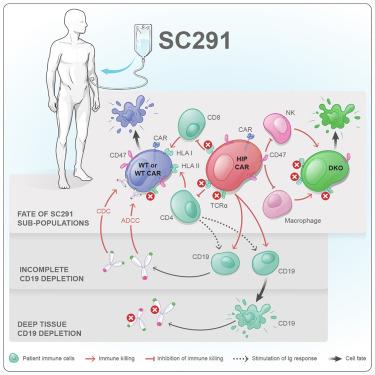
低免疫CD19 CAR - T细胞逃避癌症和自身免疫性疾病患者的同种异体排斥反应
现成的CAR - T细胞需要可靠地逃脱同种异体免疫反应,才能成为通用药物。原代T细胞产物SC291通过CD19 CAR、T细胞受体α常数(TRAC)敲除、HLA缺失和CD47过表达的低免疫(HIP)编辑进行工程化。在这里,我们报告了来自hip -编辑CD19 CAR - T细胞的ARDENT (NCT05878184)和GLEAM (NCT06294236)试验的探索性免疫分析。尽管存在针对hla -充满的SC291亚群的同种免疫反应,但我们观察到,在所有患者中,无论剂量或患者的疾病如何,都没有针对完全编辑的HIP CAR - T细胞的新生免疫反应。缺乏针对hla -充满的CAR - T细胞的抗体被认为是深层组织CD19细胞耗竭的标志,所有60天没有这种抗体的患者外周血中都伴有B细胞耗竭。所提出的免疫数据支持HIP概念避免同种异体排斥反应的可靠性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


