Generation of fluorescent and bioluminescent induced pluripotent stem cells with their application in tracking organoid development
IF 4.6
2区 医学
Q2 MEDICINE, RESEARCH & EXPERIMENTAL
Nanomedicine : nanotechnology, biology, and medicine
Pub Date : 2025-08-09
DOI:10.1016/j.nano.2025.102849
引用次数: 0
Abstract
Islet organoids hold significant promise as a renewable source of insulin-producing cells for diabetes therapy; however, an efficient system for real-time tracking and dynamic capture of the developmental processes of islet organoids remains underdeveloped. Here, we report the generation of induced pluripotent stem cells (iPSCs) stably expressing enhanced green fluorescent protein (EGFP) and luciferase (Luc) via rational plasmid construction and lentiviral transduction. Using fluorescence and bioluminescence imaging, we systematically monitored the differentiation of these EGFP/Luc-iPSCs into islet organoids, demonstrating that the reporter iPSCs maintained pluripotency, stable fluorescent/bioluminescent signals, and uncompromised differentiation potential across multiple passages. The formed islet organoids consistently exhibited robust imaging signals, enabling noninvasive visualization of their spatiotemporal developmental dynamics. Our study established an innovative imaging platform that facilitates real-time, noninvasive monitoring of islet organoid morphogenesis, provides mechanistic insights into organoid differentiation pathways, and paves the way for advancing cell-based therapeutic strategies for diabetes.
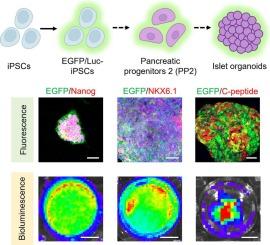
荧光和生物发光诱导多能干细胞的产生及其在类器官发育跟踪中的应用。
胰岛类器官作为胰岛素生成细胞的可再生来源,有望用于糖尿病治疗;然而,一个有效的实时跟踪和动态捕获胰岛类器官发育过程的系统仍然不发达。在这里,我们报道了通过合理的质粒构建和慢病毒转导,诱导多能干细胞(iPSCs)稳定表达增强型绿色荧光蛋白(EGFP)和荧光素酶(Luc)。利用荧光和生物发光成像,我们系统地监测了这些EGFP/Luc-iPSCs向胰岛类器官的分化,证明报告iPSCs在多个传代中保持多能性、稳定的荧光/生物发光信号和不受影响的分化潜力。形成的胰岛类器官始终表现出强大的成像信号,使其时空发育动态的无创可视化成为可能。我们的研究建立了一个创新的成像平台,促进了对胰岛类器官形态发生的实时、无创监测,提供了对类器官分化途径的机制见解,为推进基于细胞的糖尿病治疗策略铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.10
自引率
0.00%
发文量
133
审稿时长
42 days
期刊介绍:
The mission of Nanomedicine: Nanotechnology, Biology, and Medicine (Nanomedicine: NBM) is to promote the emerging interdisciplinary field of nanomedicine.
Nanomedicine: NBM is an international, peer-reviewed journal presenting novel, significant, and interdisciplinary theoretical and experimental results related to nanoscience and nanotechnology in the life and health sciences. Content includes basic, translational, and clinical research addressing diagnosis, treatment, monitoring, prediction, and prevention of diseases.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


