A novel combined lipolysis-permeation screening approach in 96-well format: Method development using type I lipid-based formulations
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
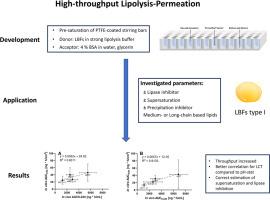
For release testing of lipid-based formulations (LBFs), lipid digestion using the pH-stat approach is widely used despite the fact that it is a laborious exercise and predictivity towards in vivo performance could not be demonstrated. A probable reason is the lack of differentiation between readily absorbable (molecularly dissolved) and less absorbable (colloid associated) drug fractions. This work describes the development and testing of an alternative approach designed to address both issues of the pH-stat method, the labor-intensive nature and the limited ability to estimate in vivo behavior. The proposed solution involves combined lipolysis-permeation testing on 96-well microtiter sandwich plates.
In this new time-efficient and material-sparing approach, a highly buffered lipolysis medium was used in the donor chamber to avoid the need for pH stabilization by titration. Moreover, the method was optimized to minimize non-specific adsorption of cinnarizine to the plates and polytetrafluoroethylene (PTFE)-coated stirring bars.
The predictive power of the new high throughput screening (HTS) to estimate in vivo oral bioavailability of cinnarizine-loaded type I LBFs was evaluated against recent oral bioavailability data of the very same formulations in rats and compared to in vitro data generated by the pH-stat lipolysis approach. The following variables were studied: supersaturation, lipase inhibition, lipid chain length, and presence of an amphiphilic polymer (precipitation inhibitor).
While the HTS method correctly captured the in vivo impact of both supersaturation and lipase inhibition for all formulations, the pH-stat method revealed opposite trends for one out of four combinations in each formulation sets. In vivo there had been no effect observed for lipid chain length nor presence of the amphiphilic polymer. In contrast, both in vitro approaches wrongly predicted such effects in some cases. A better prediction for the long-chain systems was found with the HTS method as with the laborious pH-stat approach. The HTS lipolysis-permeation method can test multiple formulations in the 96-well plate format within hours and gave IVIVCs of up to 0.91 for grouped type I LBFs. In particular the in vivo performance of supersaturated formulations was correctly captured. This study demonstrates that this new method represents a promising alternative to existing tools for prediction of the in vivo performance of type I LBFs.
一种96孔格式的新型联合溶解-渗透筛选方法:使用I型脂基配方的方法开发。
对于脂基制剂(LBFs)的释放测试,使用pH-stat方法的脂质消化被广泛使用,尽管它是一个费力的练习,并且无法证明对体内性能的预测。一个可能的原因是缺乏易吸收(分子溶解)和不易吸收(胶体相关)药物组分之间的区分。这项工作描述了一种替代方法的开发和测试,该方法旨在解决pH-stat方法的两个问题,劳动密集型的性质和有限的估计体内行为的能力。提出的解决方案包括在96孔微滴夹心板上进行联合溶解-渗透测试。在这种新的省时省材的方法中,在供体腔中使用高度缓冲的脂解培养基,以避免通过滴定来稳定pH值。此外,优化了该方法,最大限度地减少了肉桂碱对平板和聚四氟乙烯(PTFE)涂层搅拌棒的非特异性吸附。新的高通量筛选(HTS)对估计含有肉桂碱的I型lbf的体内口服生物利用度的预测能力,与最近相同配方的大鼠口服生物利用度数据进行了评估,并与pH-stat脂解方法产生的体外数据进行了比较。研究了以下变量:过饱和、脂肪酶抑制、脂质链长度和两亲性聚合物(沉淀抑制剂)的存在。虽然HTS方法正确地捕获了所有配方中过饱和和脂肪酶抑制的体内影响,但pH-stat方法在每个配方组的四分之一的组合中显示出相反的趋势。在体内,没有观察到脂链长度和两亲性聚合物的存在的影响。相比之下,在某些情况下,两种体外方法都错误地预测了这种效果。与费力的pH-stat方法相比,HTS方法对长链体系的预测效果更好。HTS溶透法可以在数小时内检测96孔板格式的多种制剂,对分组I型LBFs的ivivc高达0.91。特别是过饱和配方的体内性能被正确捕获。这项研究表明,这种新方法代表了一种有希望的替代现有工具,用于预测I型lbf的体内性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


