G2 regulatory processes require speedbump for Wee1 kinase activity
IF 2.1
3区 生物学
Q2 DEVELOPMENTAL BIOLOGY
引用次数: 0
Abstract
Wee1 is a conserved Cdk1 inhibitory kinase operating at the G2/M checkpoint to prevent entry into mitosis until the genome has been surveilled and replication is complete. We report here that the early arrest mutant speedbump is a loss-of-function mutation in the zebrafish ortholog of wee1. Like other creatures lacking Wee1 kinase, cells in the mutant enter mitosis early. Eventually, mutant cells exhibit chromosomal defects and undergo apoptosis. Live recordings of the mutant reveal that as gastrula cells transition from maternal to zygotic control, their cell cycle gets progressively shorter rather than lengthening as seen in wild-type embryos. This suggests that Wee1 kinase inhibition is part of a mechanism to slow the cell cycle that we posit is independent of its role in blocking entry into mitosis to prevent DNA damage. Supporting this view, we show that Wee1 kinase is also crucial for tissues that normally exit the cell cycle in the G2 phase. In the absence of Wee1 kinase, hatching gland cells, which typically cease dividing before speedbump defects appear, no longer remain in G2, and instead advance into mitosis before prematurely dying. Finally, we demonstrate that Wee1 kinase is essential for the endoreplication cycle in the yolk cell. We show that wild-type yolk cell nuclei transition to an S and G endocycle after they cease mitosis in the blastula. However, without Wee1 kinase these nuclei have difficulty attaining this endocycle and sometimes regress back into mitosis. We conclude that besides the regulation of mitotic timing, Wee1 kinase has other G2 regulatory roles not previously reported in which controlling entry into mitosis must be coordinated with other cellular processes.
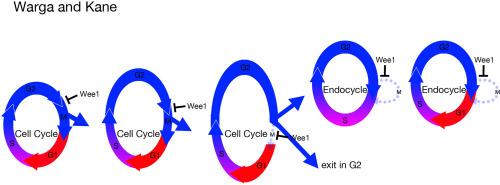
G2调节过程需要加快Wee1激酶活性。
Wee1是一种保守的Cdk1抑制激酶,在G2/M检查点起作用,防止进入有丝分裂,直到基因组被监视和复制完成。我们在这里报道,早期停止突变的速度颠簸是在斑马鱼的同源wee1的功能丧失突变。像其他缺乏Wee1激酶的生物一样,突变体中的细胞很早就进入有丝分裂。最终,突变细胞表现出染色体缺陷并发生凋亡。突变体的实时记录显示,当原肠细胞从母体控制转变为合子控制时,它们的细胞周期逐渐变短,而不是像野生型胚胎那样变长。这表明,Wee1激酶抑制是减缓细胞周期机制的一部分,我们认为这一机制独立于其阻断有丝分裂以防止DNA损伤的作用。为了支持这一观点,我们发现Wee1激酶对于通常在G2期退出细胞周期的组织也是至关重要的。在缺乏Wee1激酶的情况下,通常在速度突缺陷出现之前停止分裂的孵化腺细胞不再停留在G2中,而是在过早死亡之前进入有丝分裂。最后,我们证明了Wee1激酶对卵黄细胞的内复制周期至关重要。我们发现野生型卵黄细胞核在囊胚中停止有丝分裂后转变为S和G内环。然而,如果没有Wee1激酶,这些细胞核很难进入内循环,有时会退回到有丝分裂。我们得出结论,除了对有丝分裂时间的调节外,Wee1激酶还具有其他G2调节作用,这些作用以前没有报道过,其中控制进入有丝分裂必须与其他细胞过程协调。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Developmental biology
生物-发育生物学
CiteScore
5.30
自引率
3.70%
发文量
182
审稿时长
1.5 months
期刊介绍:
Developmental Biology (DB) publishes original research on mechanisms of development, differentiation, and growth in animals and plants at the molecular, cellular, genetic and evolutionary levels. Areas of particular emphasis include transcriptional control mechanisms, embryonic patterning, cell-cell interactions, growth factors and signal transduction, and regulatory hierarchies in developing plants and animals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


