Identification and characterization of the multifunctional glycosyltransferase AdUGT86A1 in Angelica decursiva
IF 6.2
1区 农林科学
Q1 AGRICULTURAL ENGINEERING
引用次数: 0
Abstract
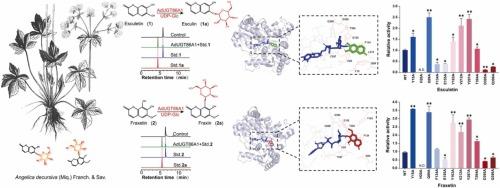
Based on the limited substrate diversity of known coumarin glycosyltransferases, we identified and characterized AdUGT86A1, a novel O-glycosyltransferase from Angelica decursiva. In vitro enzyme activity assay and substrate promiscuity analysis showed that AdUGT86A1 catalyzed the glycosylation of various coumarin derivatives (e.g., esculetin and fraxetin) and certain flavonoids (e.g., tectorigenin) to produce the corresponding glycosides. Enzymological characterization revealed that AdUGT86A1 exhibited optimal catalytic activity at a pH of 7.0 and 30°C, with Ca2+ ions significantly enhancing the catalytic activity. Homology modeling and molecular docking showed that AdUGT86A1 had a GT-B fold architecture with substrate-binding patterns, with H20, Q99, Y163, V212, and Y297 serving as crucial residues for its catalytic function. Notably, alanine substitution at Y297 and Q99 increased the yields of esculin and fraxin by 2–3 fold, respectively. AdUGT86A1 represents not only an efficient biocatalytic tool for coumarin glycoside biosynthesis but also a molecular template for engineering multifunctional glycosyltransferases owing to its unique substrate promiscuity. This study offers a significant reference for enhancing natural product structural modification and drug development.
当归多功能糖基转移酶AdUGT86A1的鉴定与表征
基于已知香豆素糖基转移酶有限的底物多样性,我们从当归中鉴定并鉴定了一种新的o -糖基转移酶AdUGT86A1。体外酶活性测定和底物混杂性分析表明,AdUGT86A1可催化多种香豆素衍生物(如槲皮素和黄曲霉素)和某些黄酮类化合物(如鸢尾黄素)的糖基化,生成相应的糖苷。酶学表征表明,AdUGT86A1在pH 7.0和30℃条件下具有最佳的催化活性,Ca2+离子显著增强了AdUGT86A1的催化活性。同源性建模和分子对接表明,AdUGT86A1具有GT-B折叠结构,具有底物结合模式,其中H20、Q99、Y163、V212和Y297是其催化功能的关键残基。值得注意的是,在Y297和Q99上替换丙氨酸分别使esculin和fraxin的产量提高了2-3倍。AdUGT86A1不仅是香豆素糖苷生物合成的高效生物催化工具,而且由于其独特的底物混杂性,也成为工程多功能糖基转移酶的分子模板。该研究为加强天然产物结构修饰和药物开发提供了重要参考。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial Crops and Products
农林科学-农业工程
CiteScore
9.50
自引率
8.50%
发文量
1518
审稿时长
43 days
期刊介绍:
Industrial Crops and Products is an International Journal publishing academic and industrial research on industrial (defined as non-food/non-feed) crops and products. Papers concern both crop-oriented and bio-based materials from crops-oriented research, and should be of interest to an international audience, hypothesis driven, and where comparisons are made statistics performed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


