Tumor-associated Schwann cell remodeling under metabolic stress via lactate sensing orchestrates pancreatic ductal adenocarcinoma development
IF 30.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Diabetes mellitus (DM) is a known risk factor for pancreatic cancer, but the underlying mechanisms remain elusive. Here, we identify lactate-driven remodeling of tumor-associated Schwann cells (TASCs) as a key mediator of immunosuppression in diabetic pancreatic ductal adenocarcinoma (PDAC). Single-cell RNA sequencing revealed a c1-Mettl16+Cd276+Nectin2+ TASC subpopulation enriched in diabetic tumors that impairs CD8+ T cell function and promotes PD-1 resistance. Mechanistically, lactate enters TASCs via MCT1/MCT4, binds METTL16, and induces K269 lactylation, enhancing m6A-dependent CTCF stabilization and transcriptional activation of immunosuppressive ligands. Targeting METTL16 restores immune surveillance and sensitizes tumors to PD-1 blockade. Retrospective analyses confirmed therapeutic benefit in patients with diabetic PDAC receiving rosuvastatin. These findings uncover a lactate-METTL16-CTCF axis that links metabolic stress to epitranscriptomic reprogramming and immune evasion, offering a promising strategy to potentiate immunotherapy in metabolically dysregulated PDAC.
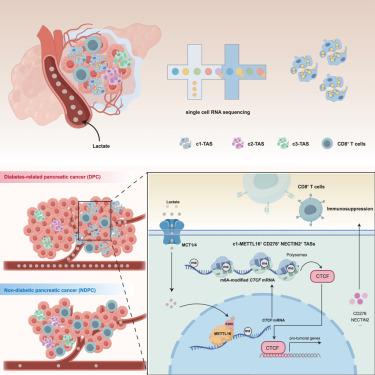
代谢应激下通过乳酸感知介导的肿瘤相关雪旺细胞重塑调控了胰腺导管腺癌的发展
糖尿病(DM)是胰腺癌的已知危险因素,但其潜在机制尚不清楚。在这里,我们发现乳酸驱动的肿瘤相关雪旺细胞(TASCs)重塑是糖尿病胰腺导管腺癌(PDAC)免疫抑制的关键介质。单细胞RNA测序显示,c1-Mettl16+Cd276+Nectin2+ TASC亚群在糖尿病肿瘤中富集,损害CD8+ T细胞功能并促进PD-1抵抗。在机制上,乳酸通过MCT1/MCT4进入TASCs,结合METTL16,诱导K269乳酸化,增强m6a依赖性CTCF的稳定性和免疫抑制配体的转录激活。靶向METTL16恢复免疫监视并使肿瘤对PD-1阻断敏感。回顾性分析证实了接受瑞舒伐他汀治疗的糖尿病PDAC患者的治疗效果。这些发现揭示了一个乳酸- mettl16 - ctcf轴,它将代谢应激与表转录组重编程和免疫逃避联系起来,为加强代谢失调的PDAC的免疫治疗提供了一个有希望的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


