PPh2H-involving mixed ligands-controlled Rh-catalyzed iso-selective hydroformylation of 1,3-butadiene
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The hydroformylation of olefins is a cornerstone of homogeneous catalysis and industrial chemistry, yet achieving precise regioselectivity, particularly for 1,3-butadiene, remains a formidable challenge. Here we introduce a transformative rhodium-based catalytic system that achieves unprecedented efficiency in the hydroformylation of 1,3-butadiene, yielding dialdehydes with exceptional efficiency (up to 88 %). Our strategy employs a mixed dual-ligand system combining a tetradentate phosphine ligand and a diphenylphosphane, which synergistically facilitate the two-stage hydroformylation process during the catalytic cycle of CO insertion. This cooperative function of mixed ligands enables the selective production of 2-ethylbutanedial with a record-breaking iso-selectivity of up to 67.8 %. Mechanistic investigations, supported by computational and experimental studies, reveal the critical and competitive role of binary mixed-ligand catalysis (BMLC) and ligand-relay catalysis (LRC) in this reaction. Specifically, three coordination modes derived from multidentate phosphine and monodentate diphenylphosphane ligands alternately govern key steps of the catalytic cycle, including olefin activation and regioselective insertion.
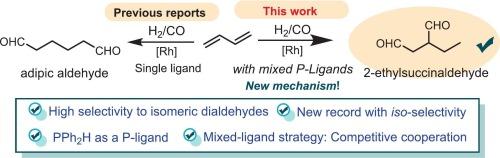
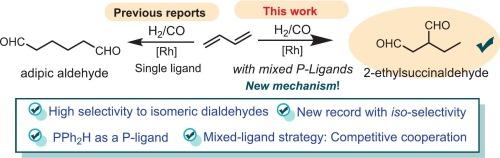
涉及混合配体的pph2h控制的铑催化的1,3-丁二烯的等选择性氢甲酰化
烯烃的氢甲酰化反应是均相催化和工业化学的基础,但实现精确的区域选择性,特别是1,3-丁二烯的区域选择性,仍然是一个艰巨的挑战。在这里,我们介绍了一种变革性的铑基催化系统,该系统在1,3-丁二烯的氢甲酰化反应中实现了前所未有的效率,产生了特殊的二醛(高达88%)。我们的策略采用混合双配体体系,结合四齿膦配体和二苯基膦,在CO插入的催化循环中协同促进两阶段氢甲酰化过程。这种混合配体的协同功能使得2-乙基丁二醛的选择性生产具有破纪录的同位选择性高达67.8%。在计算和实验研究的支持下,机理研究揭示了二元混合配体催化(BMLC)和配体接力催化(LRC)在该反应中的关键和竞争性作用。具体来说,由多齿膦和单齿二苯基膦配体衍生的三种配位模式交替控制催化循环的关键步骤,包括烯烃活化和区域选择性插入。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


