LCCPs exposure induces pulmonary inflammatory injury via AIM2 mediated pulmonary macrophage PANoptosis and M1 polarization
IF 7.3
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
In recent years, with the wide application of long-chain chlorinated paraffins (LCCPs), long-chain chlorinated paraffins have been detected all over the world. High concentrations of LCCPs are being detected in the air of human living environments. As the main gas exchange organ of the human body, the lungs are responsible for transporting oxygen throughout the body, but there are no studies on the toxicological effects of LCCPs on the lungs. In this study, in vitro and in vivo models were used to investigate the toxicological damage of LCCPs to the lungs and its potential mechanism. In vitro, this study found that LCCPs induced mitochondrial damage and oxidative stress in alveolar epithelial cells (AECs) and alveolar macrophages (AMs), and promoted apoptosis in AECs as well as PANoptosis in AMs. Molecular mechanism studies have revealed that mtDNA leakage into the cytoplasm through VDAC protein channels activated AIM2 to assemble inflammasomes, which in turn triggered the cellular innate immune response. At the same time, AIM2 activation triggered a macrophage phenotype towards pro-inflammatory (M1) polarization, reducing macrophage phagocytosis and repair. In vivo, alveolar macrophages were found to be the key regulators of LCCPs-induced pneumonia, and the upregulation of AIM2 led to the secretion of pro-inflammatory cytokines by alveolar macrophages, which recruited neutrophils to the alveoli, along with the loss of their ability to clear dead cells and to repair tissue damage. The pro-inflammatory response of macrophages exacerbates the alveolar homeostatic imbalance, ultimately leading to AIM2-dependent lung inflammation. The results of this study indicate that LCCPs may have a harmful effect in promoting pneumonia, suggesting that humans need to control the use of LCCPs in the future.
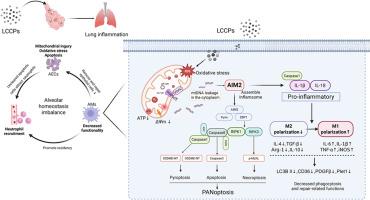
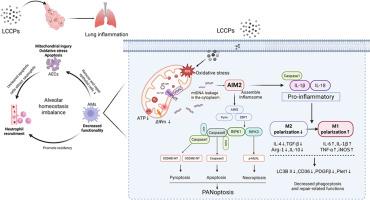
LCCPs暴露通过AIM2介导的肺巨噬细胞PANoptosis和M1极化诱导炎性损伤
近年来,随着长链氯化石蜡(LCCPs)的广泛应用,长链氯化石蜡在世界各地都有检测到。在人类生活环境的空气中检测到高浓度的LCCPs。肺是人体的主要气体交换器官,负责将氧气输送到全身,但目前还没有关于LCCPs对肺的毒理学影响的研究。本研究采用体外和体内模型,探讨LCCPs对肺的毒理学损害及其潜在机制。本研究在体外发现,LCCPs诱导肺泡上皮细胞(AECs)和肺泡巨噬细胞(AMs)线粒体损伤和氧化应激,促进AECs细胞凋亡和AMs细胞PANoptosis。分子机制研究表明,mtDNA通过VDAC蛋白通道渗漏到细胞质中,激活AIM2组装炎性小体,进而引发细胞先天免疫反应。同时,AIM2激活引发巨噬细胞向促炎(M1)极化的表型,减少巨噬细胞的吞噬和修复。在体内,肺泡巨噬细胞被发现是lccps诱导的肺炎的关键调节因子,AIM2的上调导致肺泡巨噬细胞分泌促炎细胞因子,将中性粒细胞招募到肺泡,同时丧失清除死细胞和修复组织损伤的能力。巨噬细胞的促炎反应加剧了肺泡内稳态失衡,最终导致aim2依赖性肺部炎症。本研究结果提示,LCCPs可能在促进肺炎方面具有有害作用,提示人类未来需要控制LCCPs的使用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environmental Pollution
环境科学-环境科学
CiteScore
16.00
自引率
6.70%
发文量
2082
审稿时长
2.9 months
期刊介绍:
Environmental Pollution is an international peer-reviewed journal that publishes high-quality research papers and review articles covering all aspects of environmental pollution and its impacts on ecosystems and human health.
Subject areas include, but are not limited to:
• Sources and occurrences of pollutants that are clearly defined and measured in environmental compartments, food and food-related items, and human bodies;
• Interlinks between contaminant exposure and biological, ecological, and human health effects, including those of climate change;
• Contaminants of emerging concerns (including but not limited to antibiotic resistant microorganisms or genes, microplastics/nanoplastics, electronic wastes, light, and noise) and/or their biological, ecological, or human health effects;
• Laboratory and field studies on the remediation/mitigation of environmental pollution via new techniques and with clear links to biological, ecological, or human health effects;
• Modeling of pollution processes, patterns, or trends that is of clear environmental and/or human health interest;
• New techniques that measure and examine environmental occurrences, transport, behavior, and effects of pollutants within the environment or the laboratory, provided that they can be clearly used to address problems within regional or global environmental compartments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


