Targeting the MYC oncogene with a selective bi-steric mTORC1 inhibitor elicits tumor regression in MYC-driven cancers
IF 7.2
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
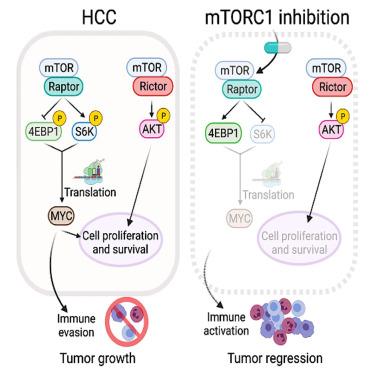
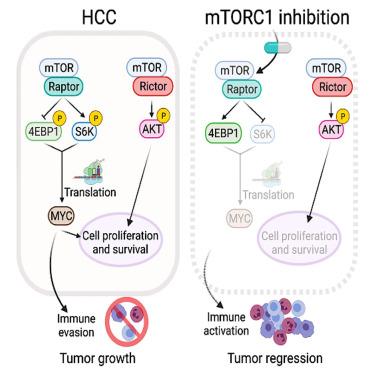
The MYC oncogene is causally involved in the pathogenesis of most human cancers. The mTORC1 complex regulates MYC translation through 4EBP1 and S6K. However, agents that selectively target mTORC1 (without affecting mTORC2) have so far failed to reactivate 4EBP1 and, thus, cannot effectively suppress MYC in vivo. In contrast, nonselective inhibitors that block both mTOR complexes can activate 4EBP1, but often lack tolerability and induce immunosuppression. Here, we introduce bi-steric mTORC1-selective inhibitors, including the clinical candidate RMC-5552, which potently reactivate 4EBP1 and decrease MYC protein expression levels. Consequently, suppression of MYC signaling occurs, resulting in tumor growth inhibition through both direct effects on tumor cells and immune activation. RMC-5552 exhibits anti-tumor activity in human patient-derived xenografts models harboring genomic MYC amplifications and reduces MYC protein levels in vivo. Furthermore, bi-steric mTORC1-selective inhibitors enhance the efficacy of immune checkpoint blockade, leading to tumor regression.
用选择性双位mTORC1抑制剂靶向MYC癌基因可诱导MYC驱动型癌症的肿瘤消退
MYC癌基因与大多数人类癌症的发病机制有因果关系。mTORC1复合体通过4EBP1和S6K调控MYC翻译。然而,选择性靶向mTORC1(不影响mTORC2)的药物迄今未能重新激活4EBP1,因此不能在体内有效抑制MYC。相比之下,阻断两种mTOR复合物的非选择性抑制剂可以激活4EBP1,但通常缺乏耐受性并诱导免疫抑制。在这里,我们引入了双位mtorc1选择性抑制剂,包括临床候选rmmc -5552,它能有效地重新激活4EBP1并降低MYC蛋白表达水平。因此,MYC信号被抑制,通过对肿瘤细胞的直接作用和免疫激活抑制肿瘤生长。rmmc -5552在含有基因组MYC扩增的人类患者来源的异种移植物模型中显示出抗肿瘤活性,并在体内降低MYC蛋白水平。此外,双位mtorc1选择性抑制剂增强免疫检查点阻断的功效,导致肿瘤消退。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


