Peripheral neuronal sensitization and neurovascular remodelling in osteoarthritis pain
IF 32.7
1区 医学
Q1 RHEUMATOLOGY
引用次数: 0
Abstract
Pain is the primary complaint in individuals with osteoarthritis (OA) and changes as the disease progresses. Anatomical changes in several joint structures potentially contribute to pain, including the increased innervation of the periosteum, synovium and subchondral bone, and the pathological innervation of articular cartilage, which is aneural under physiological conditions. Research has focused on molecules that sensitize afferent neurons, such as neuropeptides, neurotrophins, pro-inflammatory cytokines and ion channels. The neurotrophin nerve growth factor (NGF) is the best validated target in OA pain, with proven analgesic effects in preclinical and clinical studies, although the development of NGF-targeted therapeutics has been hampered by serious side effects. One relatively neglected area of research is the contribution to OA pain of the molecular pathways that mediate remodelling of nerves in disease. Remodelling requires coordination between the nerve and the associated vasculature, along with signals that are received from the surrounding parenchyma. Key cell guidance molecules, including angiogenic factors, ephrins, semaphorins and SLIT proteins are involved in nerve growth during development, and their expression is increased in osteoarthritic joints. Peripheral mechanisms of pain in osteoarthritis include nociceptor sensitization via the function of ion channels and pro-inflammatory molecules, and, potentially, pathways supporting neuronal growth and differentiation within the diseased joint. This Review discusses how neuronal trophism and neurovascular remodelling could be targeted in combination with neuronal de-sensitization or joint re-structuring approaches to reduce osteoarthritic pain.

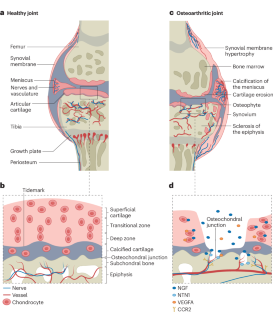
骨关节炎疼痛的周围神经敏化和神经血管重构
疼痛是骨关节炎(OA)患者的主要主诉,并随着疾病的进展而变化。几种关节结构的解剖改变可能导致疼痛,包括骨膜、滑膜和软骨下骨的神经支配增加,以及关节软骨的病理神经支配,在生理条件下是神经的。研究主要集中在使传入神经元敏感的分子上,如神经肽、神经营养因子、促炎细胞因子和离子通道。神经营养因子神经生长因子(NGF)是治疗OA疼痛的最佳靶点,在临床前和临床研究中证实了其镇痛作用,尽管NGF靶向治疗的发展一直受到严重副作用的阻碍。一个相对被忽视的研究领域是疾病中介导神经重塑的分子通路对OA疼痛的贡献。重建需要神经和相关的脉管系统之间的协调,以及从周围实质接收的信号。血管生成因子、ephrins、信号蛋白、SLIT蛋白等关键细胞引导分子参与神经发育过程,在骨关节炎关节中表达增加。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Rheumatology
医学-风湿病学
CiteScore
29.90
自引率
0.90%
发文量
137
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Rheumatology is part of the Nature Reviews portfolio of journals. The journal scope covers the entire spectrum of rheumatology research. We ensure that our articles are accessible to the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


